Gemtuzumab Effective in AML Pts Over 60 in First Relapse
SAN DIEGO-Three multicenter phase II trials in the United States, Canada, and Europe, representing the pivotal trials that led to approval of gemtuzumab ozogamicin (Mylotarg), were updated at the 45th Annual Meeting of the American Society of Hematology (abstract 615), looking only at patients age 60 and over. Richard A. Larson, MD, professor of medicine, University of Chicago, presented the results of the largest series of older patients with acute myeloid leukemia (AML) in first relapse who have been treated with gemtuzumab.
SAN DIEGOThree multicenter phase II trials in the United States, Canada, and Europe, representing the pivotal trials that led to approval of gemtuzumab ozogamicin (Mylotarg), were updated at the 45th Annual Meeting of the American Society of Hematology (abstract 615), looking only at patients age 60 and over. Richard A. Larson, MD, professor of medicine, University of Chicago, presented the results of the largest series of older patients with acute myeloid leukemia (AML) in first relapse who have been treated with gemtuzumab.
Gemtuzumab is an antibody-targeted chemotherapy that has been FDA approved for treatment of patients 60 years of age or older with CD33-positive AML in first relapse who are not considered candidates for other cytotoxic therapy.
Dr. Larson said that 157 older AML patients in first relapse were entered into the three phase II studies. Of the 157 patients, 66 (42%), were women and 91 (58%) were men. Their ages ranged from 60 to 87 (median, 68 years). Duration of first remission was 2 to 74 months (median, 9.9 months). About half (52%) of the patients had prior high-dose cytarabine consolidation therapy.
In these studies, gemtuzumab was administered intravenously at a dose of 9 mg/m2 on days 1 and 15. All 157 patients received the first dose, 119 received both doses, and 4 received a third dose.
Study Results
Of the 157 study patients, 38 (24%) had a remission, characterized by 5% or less leukemic blast cells in the bone marrow, hemoglobin of 9 g/dL or more, absolute neutrophil count (ANC) of 1,500/µL or more, and red blood cell and platelet transfusion independence.
Dr. Larson reported that 19 of these 38 responding patients had a complete remission (CR) that included the above criteria plus recovery of platelets to 100,000/µL; another 19 patients had remission with incomplete platelet recovery (CRp).
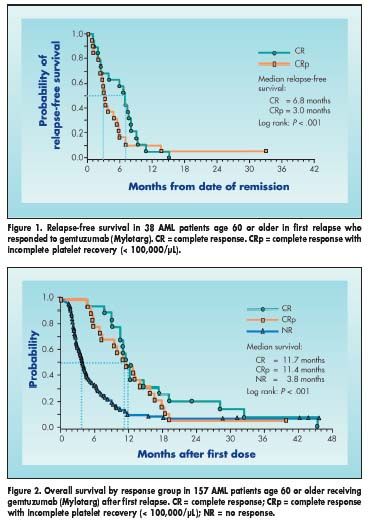
Remission response was 9% for patients with an initial duration of first complete remission of less than 6 months, 21% for 6 to 12 months, and 35% for 12 months or longer. Median relapse-free survival for complete responders was 6.8 months (Figure 1). Median overall survival for those achieving a complete response was 11.7 months (Figure 2).
"We found that patients with long first remissions are more likely to respond to this therapy," Dr. Larson commented. "But, unfortunately, the duration of remission is short."
Toxicity
The most commonly reported NCI grade 3-4 infusion toxicities were chills (11%), dyspnea (12%), nausea and vomiting (12%), and fever (13%). Dr. Larson noted that grade 3-4 mucositis occurred in five patients (3%), and sepsis and pneumonia occurred in 15% and 8% of patients, respectively. The frequency of all infections, regardless of NCI grade, was 29%. Grade 3-4 hepatic toxicities included AST elevations (16%), ALT elevations (8%), and hyperbilirubinemia (29%).
Twenty-seven patients (17%) died during the induction period. Two patients (1%), one age 73 and the other age 74, developed fatal hepatic veno-occlusive disease (VOD) after gemtuzumab treatment and before other therapy. Eleven patients received hematopoietic stem cell transplantation after gemtuzumab therapy, and one 65-year-old patient developed VOD that resolved.
Dr. Larson said that further studies are needed to evaluate gemtuzumab’s effectiveness as primary therapy or in combination with other antileukemia agents, and to determine the mechanisms of leukemia cell resistance.