Gynecologic Manifestations of Hereditary Nonpolyposis Colorectal Cancer
Hereditary nonpolyposis colorectal cancer (HNPCC) is an autosomaldominant cancer susceptibility syndrome associated with inheriteddefects in the DNA mismatch repair system. HNPCC family membersare at high risk for developing colorectal, endometrial, and ovariancancers. Studies of HNPCC families have helped define the importantrole that mismatch repair genes play in the molecular pathogenesis ofendometrial and ovarian cancers. This review will describe some of theimportant clinical and molecular features of HNPCC-related endometrialand ovarian cancer and describe how genetic susceptibility can beidentified in patients with sporadic endometrial and ovarian cancers. Itis important to identify patients with HNPCC, as families of mutationcarriers may benefit from genetic counseling, testing, and intensifiedcancer surveillance.
Hereditary nonpolyposis colorectal cancer (HNPCC) is an autosomal dominant cancer susceptibility syndrome associated with inherited defects in the DNA mismatch repair system. HNPCC family members are at high risk for developing colorectal, endometrial, and ovarian cancers. Studies of HNPCC families have helped define the important role that mismatch repair genes play in the molecular pathogenesis of endometrial and ovarian cancers. This review will describe some of the important clinical and molecular features of HNPCC-related endometrial and ovarian cancer and describe how genetic susceptibility can be identified in patients with sporadic endometrial and ovarian cancers. It is important to identify patients with HNPCC, as families of mutation carriers may benefit from genetic counseling, testing, and intensified cancer surveillance.
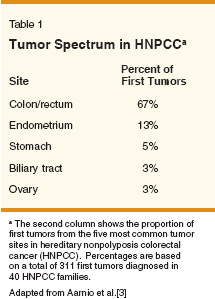
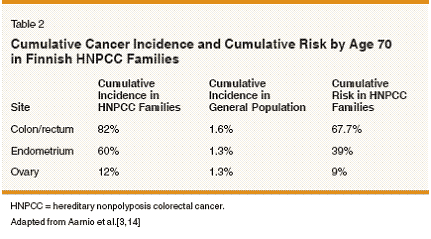
Hereditary nonpolyposis colorectal cancer (HNPCC) is an autosomal dominant cancer susceptibility syndrome associated with inherited defects in the DNA mismatch repair system. Initially described by Warthin in 1913 as the cancer family syndrome,[1] HNPCC is characterized by genetic susceptibility to multiple malignancies, most commonly of the colon and endometrium (Table 1).[2,3] HNPCC is believed to account for approximately 1% to 5% of all colorectal cancers[4] and an estimated 0.5% to 1.4% of all endometrial cancers.[5,6] The clinical features of HNPCC include earlyonset cancers, synchronous and/or metachronous malignancies, and a nearly complete (80%) penetrance of the disease. Lifetime risk for cancer in patients with inherited DNA mismatch repair mutations has been estimated as being as high as 91% for males and 69% for females.[7] As shown in Table 2, the cumulative incidence of colorectal, endometrial, and ovarian cancer in HNPCC is significantly higher than in the general population. The majority (70%-80%) of HNPCC cases result from germline mutations in the DNA mismatch repair genes MLH1 and MSH2.[8,9] Germline mutations in other mismatch repair genes MSH6, PMS1, PMS2 are found less frequently.[8] Loss of the normal DNA mismatch repair leads to genomic instability and confers a mutator phenotype.[10,11] The mutator phenotype is an essential feature of tumorigenesis. Mismatch repair- deficient cells rapidly accumulate somatic mutations[12] that can affect a variety of target genes regulating cell growth and/or apoptosis. Mutations in DNA mismatch repair- deficient cells are typically single- base mismatches and insertion/ deletions that frequently occur within repeated DNA sequences. Tumors deficient in mismatch repair often exhibit instability in microsatellite DNA repeats. This tumor phenotype is called microsatellite instability (MSI). The MSI phenotype is observed in a high proportion (80%-90%) of colorectal cancers in patients with inherited (germline) mutations in MLH1 or MSH2.[13] Since endometrial and ovarian cancer frequently occur in HNPCC families,[3,14-17] it is not surprising that defective mismatch repair plays a significant role in sporadic endometrial and ovarian tumorigenesis. Clinical diagnosis of HNPCC relies on family history, and multiple criteria have been employed to identify putative carriers of mismatch repair gene defects (Table 3). The criteria have been expanded over time to include extracolonic malignancies as well as other risk factors for genetic disease such as synchronous or metachronous tumors. Identification of HNPCC is important because families of mutation carriers may benefit from genetic counseling, testing, and intensified cancer surveillance.
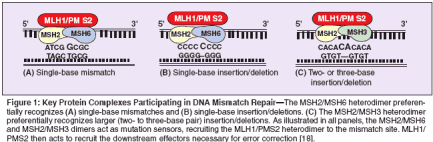
This review will discuss the molecular basis of HNPCC-related malignancies and will specifically focus on the clinical relevance of mismatch repair deficiency in sporadic and inherited forms of endometrial and ovarian cancer. Molecular Biology of Mismatch Repair Mismatch repair genes function to ensure fidelity of DNA replication in cell division. Replication errors can occur through mismatches produced by physical or chemical alteration of nucleotides, misincorporation of nu cleotides during replication, and genetic recombination events. There are now seven mismatch repair genes that have been investigated in cancers: MLH1, MSH2, MSH3, MSH6, PMS1, PMS2, and MLH3. Figure 1 illustrates how these proteins normally function as complexes to repair single-base mismatches or insertion/deletion type mutations. The fact that the majority of MSI-positive tumors have MLH1 or MSH2 defects supports the unique functionality of MLH1 and MSH2. The MSH2/MSH6 and MSH2/MSH3 heterodimers function as sensors, recognizing mismatched DNA, whereas the MLH1/PMS2 heterodimer initiates correction.[18]
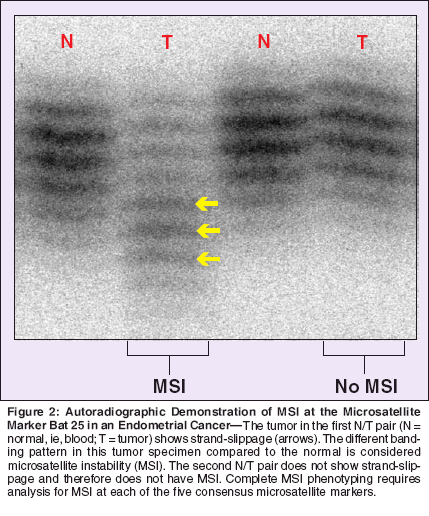
Studies of mismatch repair gene knockout mice have provided evidence for the importance of an intact DNA repair system in protecting against cancer. Colon tumors form in MSH2 null (both alleles deleted) mice and demonstrate MSI.[19] By contrast, MSH6 null mice tend to develop tumors later in life, and these tumors do not exhibit MSI.[20] Partial functional redundancy between the MSH2/MSH6 heterodimer and the MSH2/MSH3 heterodimer explains the low level of MSI found in MSH3 and MSH6 mutants.[21] Accumulation of point mutations and insertion/deletion (frameshift) mutations in repeated DNA sequences is detectable as MSI. Tumors demonstrating MSI (mismatch repair deficiency) show different polymerase chain reaction (PCR) products at multiple microsatellite sequences when compared to the normal (nontumor) DNA from the same patient (Figure 2). A consensus panel of five microsatellite markers has been established (D2S123, D5S346, D17S250, Bat 25, and Bat 26)[13] to facilitate and standardize identification of tumors that demonstrate MSI. Mechanisms of Cancer Formation
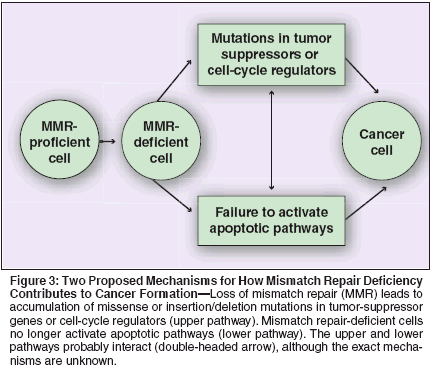
Mismatch repair deficiency contributes to cancer formation through two distinct pathways (Figure 3, upper pathway). These pathways are likely related to each other; however, a well-defined molecular link between the two mechanisms has yet to be defined. In mismatch repair-deficient endometrial cancers, tumor-suppressor genes that contain coding sequence repeats such as PTEN (phosphatase and tensin homolog), BAX (BCL2- associated X protein), and IGF2R (insulin- like growth factor 2 receptor) may preferentially acquire mutations and ultimately lead to tumor formation.[ 22-24] With the exception of PTEN (which has been shown to be mutated in approximately 85% of hereditary endometrial cancers),[25] mutations in tumor suppressors and cell-cycle regulatory proteins in MSIpositive endometrial cancers are infrequent.[ 22,24,26] The alternative mechanism for how loss of DNA repair contributes to tumorigenesis (Figure 3, lower pathway) is that cells deficient in mismatch repair fail to activate apoptotic pathways in spite of overwhelming DNA damage.[27] Cell line studies provide evidence for the role of mismatch repair proteins in the cell death pathway. MSH2 null mouse embryonic fibroblasts do not undergo apoptosis in response to the DNA-damaging agent MNNG (N-methyl-N'-nitro-Nnitrosoguanidine), and overexpression of MSH2 or MLH1 can induce (rescue) apoptosis in MSI-positive or MSI-negative cells.[28] Tumor cell selection may therefore be initiated by a failure of defective mismatch repair proteins (MLH1 and MSH2) to stimulate existing apoptotic pathways.[ 27] As noted, MSI in hereditary cancer is associated with an underlying defect in one of the DNA mismatch repair genes. Sporadic (nonfamilial) endometrial tumors, however, rarely demonstrate mutations in these genes.[29-32] In sporadic endometrial cancers, it is much more common for MLH1 to be inactivated epigenetically through promoter hypermethylation. An estimated 70% to 90% of endometrial cancers with MSI demonstrate aberrant methylation of the MLH1 promoter.[33,34] Promoter hypermethylation in endometrial cancer is associated with loss of MLH1 expression.[35] The elevated cancer risk associated with the presence of both germline (HNPCC) coupled with the frequent somatic (epigenetic methylation) inactivation of the mismatch repair genes (MLH1) highlights the significance of these genes in tumorigenesis. Endometrial CancerGenetic Susceptibility
Approximately 5% of all endometrial cancers result from inherited cancer susceptibility.[36] Of inherited endometrial cancers, an estimated 0.5% to 1.4%[5,6] are HNPCC-related. HNPCC is the best-understood inherited endometrial cancer susceptibility syndrome. Endometrial cancer is the most frequent extracolonic tumor in HNPCC,[2,3,17] and the cumulative lifetime risk of endometrial cancer in HNPCC families is high- approximately 40% to 60%.[3,14] Although the majority of germline mutations in HNPCC families are in MLH1 and MSH2,[8] families that carry germline mutations in MSH6 appear to have a preponderance of endometrial cancers regardless of whether or not they meet strict (Amsterdam) clinical criteria.[37,38] An analysis of 20 families with 146 MSH6 mutation carriers demonstrated an overall lower risk for all HNPCCrelated tumors but a significantly higher risk for endometrial cancer compared to MLH1 and MSH2 mutation carriers.[39]

Clinical criteria for diagnosing HNPCC have broadened over time (Table 3) to reflect the understanding that there is genetic risk in patients who do not meet strict (Amsterdam) clinical diagnostic criteria. There are at least three risk factors for having germline (inherited) defects in one of the mismatch repair genes. Genetic susceptibility is found in patients who develop endometrial cancer at a young age (< 60 years old),[36,40] who present with synchronous or metachronous malignancies,[41] or whose tumors demonstrate MSI without epigenetic silencing of MLH1 (MLH1- unmethylated tumors).[42] Women who do not meet strict clinical (Amsterdam) criteria for HNPCC but have early-onset (< 60 years old) disease appear to have increased familial clustering of malignancies. In a large phone survey of 455 women with early-onset endometrial cancer (20-54 years old) compared with over 3,000 age-matched controls, the odds ratio for endometrial cancer in a firstdegree relative was 2.8 (95% confidence interval [CI] = 1.9-4.2), and the odds ratio for colorectal cancer within the same family was 1.9 (95% CI = 1.1-3.3).[40] Another study examined 291 early-onset (< 60 years old) endometrial cancer cases with available parental data. Nine kindreds (3.1%) met clinical criteria for HNPCC, and an additional 3% had familial clustering of malignancies.[36]
Patients with double primary malignancies of the colon and endometrium are another subgroup at-risk for inherited cancer susceptibility. In a study of 40 unrelated women with colorectal cancer and endometrial cancer, 7 patients had MLH1 or MSH2 mutations and 6 of the 7 had family histories suggestive of HNPCC. The relative risk of colorectal cancer in first-degree relatives of patients who carried germline mutations in MLH1 or MSH2 was 8.1 (95% CI = 3.5- 15.9) and the relative risk of endometrial cancer was 23.8 (95% CI = 6.4-61.0). First-degree relatives of patients with double primary malignancies without MLH1 or MSH2 defects were also at increased risk for colorectal cancer or endometrial cancer, with a relative risk of 2.8 (95% CI = 1.7-4.5) and 5.4 (95% CI = 2.0- 11.7), respectively.[41] Another study of women diagnosed with both colon and endometrial cancer (N = 80) revealed that the relative risk of colorectal cancer before age 55 in first-degree relatives of probands who had both cancers before age 55 was 30.5 (95% CI = 18.8-46.6).[43] In addition to clinical factors (early- onset disease and double primary malignancies), loss of mismatch repair in endometrial tumors can be helpful in identifying women with genetic susceptibility. A molecular phenotype (MSI-positive, MLH1-unmethylated) has been identified that appears to highlight carriers of germline mismatch repair mutations. Women with MSI-positive endometrial cancer without MLH1 methylation demonstrate familial clustering of malignancies, develop cancer almost 10 years earlier,[42] and have an excess of HNPCC-associated metachronous malignancies.[44] Clinical Significance of Mismatch Repair Deficiency
Identification of HNPCC patients is important because family members have been shown to benefit significantly from enrollment in colorectal cancer surveillance programs.[45] Whether the loss of mismatch repair in HNPCC-related or sporadic endometrial cancers is related to tumor behavior or overall survival, however, is controversial. In HNPCC-related endometrial cancers, no difference has been seen in 5-year cumulative survival (all stages) or in histologic subtype compared to age- and stage-matched sporadic endometrial cancers.[46] In a small study (109 tumor samples) that compared MSI-positive (N = 10 tumors with high-level MSI) to MSI-negative sporadic endometrial cancers, MSI positivity was associated with higher tumor grade and poor clinical outcome.[47] A larger retrospective study of sporadic MSI-positive and MSI-negative endometrial cancers, however, found no difference in 5-year survival, 5-year recurrencefree survival, stage, grade, or histologic subtype.[48] Another retrospective case/control study of 29 MSI-positive endometrial cancer patients demonstrated statistically significant improvement in 5- year survival (77% vs 48%) in a cohort of patients weighted heavily towards advanced-stage disease (> 50% of the patients had International Federation of Gynecology and Obstetrics [FIGO] stage III or IV disease).[49] This benefit was seen in univariate and multivariate analysis. An analysis of 70 MSI-positive vs 159 MSI-negative endometrial cancers (81% completely surgically staged) found that MSIpositive tumors tend to present with early-stage disease and have a less aggressive histology.[50] There were also no differences seen in recurrence or survival, but the median follow-up for both groups was only 24 months.
Overall, it is important to recognize patients who may have inherited susceptibility to endometrial cancer, as family members will benefit from colorectal surveillance and may similarly benefit from gynecologic surveillance. The relationship between MSI and endometrial cancer biology and overall survival in HNPCC-related or sporadic endometrial cancer is not yet fully understood. A larger analysis including prospectively acquired clinical data in surgically staged patients with known molecular phenotypes would be helpful to better understand differences in clinical outcomes in these patients.Ovarian CancerGenetic Susceptibility
Approximately 5% to 10% of ovarian cancers are the result of inherited cancer susceptibility. BRCA1 and BRCA2 mutations account for the majority of inherited ovarian cancers.[ 51] Although less prevalent, mutations in mismatch repair genes (HNPCC) represent a clinically significant form of inherited ovarian cancer. As shown in Table 2, the cumulative incidence of ovarian cancer in patients with HNPCC is approximately 12%,[14] and there are significantly more (3.5-fold) ovarian cancers diagnosed in HNPCC families.[ 15] The lifetime risk of ovarian cancer is higher in HNPCC families than in the general population,[3] but the overall low frequency of MSI in sporadic ovarian cancers has made it difficult to identify genetic susceptibility outside of Amsterdam criteria. Defective DNA mismatch repair is a feature of inherited and sporadic ovarian cancers. MSI is seen in ovarian cancer cell lines[52] and in primary tumors. In the largest series to date, 17% of 125 unselected epithelial ovarian cancer specimens had an MSI-H phenotype (half due to epigenetic silencing of MLH1).[53] As in endome- trial cancer, there have been attempts to identify patients with genetic susceptibility who do not meet strict clinical (Amsterdam) criteria for HNPCC. Early-onset disease,[54] and synchronous primary malignancies[55] have been investigated in epithelial ovarian cancer but were not able to identify patients with genetic susceptibility outside HNPCC criteria. In a study comparing patients with a family history of cancer (N = 28) and those without (N = 62), no difference in MSI positivity was seen (11% with family history and 13% without). Furthermore, no germline mutations were identified in MLH1 or MSH2 in MSIpositive ovarian cancers.[56] In women (N = 101) with very early-onset epithelial ovarian cancer (< 30 years old), only 2% had germline mutations in MLH1 (none in MSH2) and none of those patients had family histories suggestive of HNPCC.[54] Investigation of MSI concordance in synchronous ovarian and endometrial cancers[55] has not been informative due to the low prevalence of mismatch repair mutations in ovarian cancer. Overall, it is important to know that patients in HNPCC families are at risk for developing ovarian cancer, but it has been difficult to identify genetic susceptibility in unselected ovarian cancer patients. Further characterization of mismatch repair deficiency in epithelial ovarian cancer may lead to identification of additional (outside of clinical criteria) patient populations at-risk for HNPCC. Clinical Significance of Mismatch Repair Deficiency
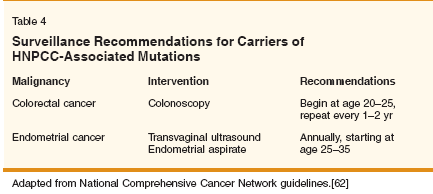
As is the case for HNPCC-related endometrial cancer, the primary goal is to identify at-risk families and ensure enrollment in surveillance programs. Retrospective analysis has shown that inherited ovarian cancers due to mutations in BRCA1 may have a favorable prognosis when compared to sporadic tumors matched for age, stage, grade, and histologic subtype.[ 57] Similar favorable clinical outcomes have been described for ovarian cancer patients from HNPCC families [58]. Ovarian cancers (N = 80) identified from HNPCC families occurred at a young age (mean = 43 years old), tended to be early-stage (85% FIGO I/II), and were low-grade (72% grade 1/2). Additionally, 21.5% of the patients had a synchronous endometrial cancer.[58] This would suggest that loss of mismatch repair has a favorable prognosis in ovarian cancer patients with HNPCC, but ascertainment bias in this study may limit the clinical utility of the data. Loss of DNA mismatch repair in sporadic ovarian cancer, on the other hand, appears (preliminarily) to be a negative prognostic indicator. In sporadic epithelial ovarian cancers, acquisition of MLH1 methylation has been observed following treatment with platinum-based chemotherapy[ 59,60] and may predict worse overall survival independent of time to progression and age.[61] Prospective clinical studies of HNPCC-related ovarian cancer patients will be required to determine whether defective DNA mismatch repair contributes significantly to tumor behavior or clinical outcome. Genetic Testing for HNPCC-Related Gynecologic Malignancies The molecular screening algorithms that are being developed for HNPCC family members relate specifically to colon tumors. The optimal molecular screening method for gynecologic malignancies within HNPCC is evolving. The established screening and surveillance guidelines for HNPCC family members have been outlined by the National Comprehensive Cancer Network (NCCN).[62] The most direct way to identify a carrier of a mismatch repair gene mutation is if there is a known familial mutation. Directed gene sequencing for the mutation of interest could then be performed. In the absence of a known familial mismatch repair gene mutation, MSI analysis of the colorectal tumor specimen may help distinguish sporadic from inherited disease. This is because HNPCC-related (inherited) colorectal cancer has a high rate of MSI (approximately 90%), compared to a 15% MSI rate in sporadic colorectal cancer (15%).[4] Gene sequencing of MLH1 or MSH2 would therefore only be applied to tumors demonstrating MSI. The molecular screening algorithms developed for colon tumors do not necessarily apply to endometrial tumors because the rate of MSI in sporadic endometrial cancers (17%-30%) is higher than that in colon cancer.[22,34,63] In HNPCC families with endometrial cancer, alternative molecular screening regimens including immunohistochemistry (IHC) to detect protein loss may be employed. One study examined the value of IHC in three groups of patients: (1) patients with endometrial cancer in HNPCC families, (2) patients in HNPCC families who underwent endometrial biopsy for nonmalignant reasons, and (3) patients at genetic risk (age < 50) without a family history suggestive of HNPCC. The authors found that 100% (6/6) of tumor specimens in the HNPCC-with- endometrial cancer group had loss of immunodetectable protein (MLH1, MS2, and/ or MSH6). They identified no relationship between loss of protein by IHC and germline mutations in MLH1 or MSH2 in the sporadic group despite finding high-level MSI in almost 32% of the sporadic tumors, indicating that IHC may be helpful in distinguishing inherited from sporadic disease.[64] In sporadic endometrial cancers, however, methylation of MLH1 is largely responsible for MSI and epigenetic silencing leads to loss of immunodetectable protein.[35] An efficient way to identify HNPCC-related endometrial cancer, therefore, may be through a combination of promoter methylation analysis and IHC. Molecular screening can be a powerful tool to identify individuals with genetic susceptibility, but it is best used in conjunction with a detailed family history. One study found 5/22 patients who gave a history of HNPCC-related malignancies in firstdegree relatives had germline mutations in MLH1, MSH2, or MSH6. No mutations were identified in patients without a family history of HNPCCrelated malignancies.[65] When considering genetic testing for HNPCC-related gene defects, re ferral to a genetic counselor is appropriate. Genetic counseling has been shown to improve compliance with screening programs[66] and can limit use of screening in patients who are mutation negative.[67] The psychological aspects of being a mutation carrier can also be addressed more extensively by an interdisciplinary approach. The advent of genetic testing has spurred concerns of "genetic discrimination"; however, it appears that fears of insurance discrimination based on genetic testing may be unfounded.[ 68,69] Surveillance for Gynecologic Malignancies in HNPCC For HNPCC-related colon cancers, genetic testing of at-risk individuals within HNPCC families has been shown to be highly beneficial to family members, as they can be enrolled in surveillance programs. In a 15-year trial that followed two cohorts of atrisk members of 22 HNPCC families, colonoscopic screening at 3-year intervals reduced the colorectal cancer rate by 62% and significantly improved survival.[45] Although the risk of developing endometrial and ovarian cancer is significantly higher than that of the general population (see also Table 2),[3,14-16] there is little evidence to support the role of gynecologic surveillance in HNPCC. Available guidelines for gynecologic surveillance of HNPCC family members are shown in Table 4 and are outlined in reference 62. Two studies have examined the role of gynecologic screening in patients from HNPCC families and/or patients with elevated genetic risk. In the first study, annual (or biennial) transvaginal ultrasound was offered to 171 women from HNPCC families and 98 from "HNPCC-like" families over a 10-year period.[70] The majority of patients (83%) had at least one scan, and no cases of endometrial cancer were detected. The second study entailed annual transvaginal ultrasound, gynecologic examination, and measurement of serum CA-125 levels on 41 women who had not undergone prior gynecologic surgery, were either mismatch repair gene mutation carriers, and/or belonged to families fulfilling Amsterdam II criteria.[71] No endometrial or ovarian cancers were diagnosed. The median age at first screening in this study was 37 years, and the median follow-up was 5 years. The fact that half the patients were younger than age 37 years limits the power of their study to find a surveillance benefit.
The differences in clinical behavior between HNPCC-related and sporadic gynecologic malignancies are incompletely understood. Therefore, it is difficult to imagine a gynecologic surveillance regimen (using current methodology) that will improve overall survival for HNPCC family members. Chemoprevention and Prophylactic Surgery Prophylactic surgery has had a major impact on the management and counseling of patients at risk for BRCA-related breast and ovarian cancer. Given the increased risk of developing endometrial and ovarian cancer (Table 2) in HNPCC, it is reasonable to assume that HNPCC patients will benefit from prophylactic surgery as well. A decision analysis model for colorectal cancer in HNPCC patients found that immediate prophylactic colectomy in a 25- year-old mutation carrier gave the greatest life expectancy (about 2 years more than that predicted by colonoscopic surveillance alone).[72] There are no data, however, to suggest that prophylactic total hysterectomy and bilateral salpingo-oophorectomy (TAH/ BSO) provides a survival benefit to women in HNPCC families. Nonetheless, current NCCN guidelines suggest that prophylactic surgery may be considered once childbearing is completed.[62] Chemoprevention based on mismatch repair status is currently being explored for colorectal cancer patients (these trials were reviewed by Annie Yu et al),[73] in light of evidence that nonsteroidal anti-inflammatory drug (NSAID) treatment reduces MSI in colorectal cancer cells deficient in mismatch repair.[74] Primary prevention trials for HNPCC-related endometrial or ovarian cancers using depot medroxyprogesterone acetate (Depo- Provera) and oral contraceptives are just getting under way.[75] Summary Studies of HNPCC families[3,14-17] have helped define the important role DNA mismatch repair genes play in the molecular pathogenesis of endometrial and ovarian cancers. Multiple criteria have been established to clinically diagnose HNPCC (Table 3), and not surprisingly, the Bethesda criteria (which are the most inclusive) are the most sensitive for identifying families that are likely to carry MLH1 or MSH2 mutations.[76] The majority of HNPCC families have germline mutations in MLH1 and MSH2.[8] However, families with endometrial cancer as the predominant tumor type (regardless of meeting Amsterdam criteria) are likely to have a germline MSH6 mutation.[ 37,38] Additional genetic susceptibility for endometrial cancer can be found in patients who develop endometrial cancer at an early age,[36,40] who have double primary malignancies of the colon and endometrium,[ 41] and whose tumors have the MSI-H unmethylated phenotype.[ 42] It has been difficult to identify additional genetic susceptibility (outside of HNPCC clinical criteria) in ovarian cancer because the prevalence of defective mismatch repair (MSI) is low in sporadic tumors. Loss of the normal DNA mismatch repair process occurs early in tumorigenesis and may be essential for cancer progression.[10,11] Cells with defective mismatch repair accumulate insertion/deletion and single-base mismatches most commonly in repeated DNA sequences and exhibit MSI. These mutational hotspots have been investigated in tumor-suppressor genes,[22-26] and overall the targets of mismatch repair-deficient cancers remain elusive. Ongoing investigation will continue to clarify the role of mismatch repair proteins in apoptotic pathways. The clinical behavior and prognosis of mismatch repair-deficient endometrial and ovarian cancers is still undetermined. It is not surprising, therefore, that gynecologic surveillance strategies in patients at genetic risk have not been fruitful.[70,71] Identification of HNPCC families is extremely important because colorectal surveillance programs have demonstrated a survival benefit.[45] Molecular screening algorithms are being refined for colorectal cancers[ 62] but do not necessarily apply to endometrial or ovarian cancers. For endometrial cancer, available evidence suggests that combining IHC with MLH1 promoter methylation analysis will be effective in identifying a cohort of patients at highest risk for genetic susceptibility. This combined molecular screening regimen for endometrial cancer has yet to be tested clinically and is not currently part of the NCCN guidelines for screening and surveillance of HNPCC families. Chemoprevention and prophylactic surgery for HNPCC are under investigation.[ 73,75] No current studies have suggested that chemoprevention or prophylactic surgery will offer improved quality of life or improved survival in patients with HNPCCrelated endometrial or ovarian cancer. Additionally, there are no consensus recommendations regarding prophylactic surgery in these patients due to a lack of sufficient evidence.[77] Despite the current lack of data, prophylactic hysterectomy and complete oophorectomy may be considered in patients who have completed childbearing.[ 62]
Disclosures:
The authors have no significant financial interest or other relationship with the manufacturers of any products or providers of any service mentioned in this article.
References:
1. Warthin A: Heredity with reference to carcinoma. Arch Intern Med 12:546-555, 1913.
2. Mecklin JP, Jarvinen HJ: Tumor spectrum in cancer family syndrome (hereditary nonpolyposis colorectal cancer). Cancer 68:1109-1112, 1991.
3. Aarnio M, Mecklin JP, Aaltonen LA, et al: Life-time risk of different cancers in hereditary non-polyposis colorectal cancer (HNPCC) syndrome. Int J Cancer 64:430-433, 1995.
4. Aaltonen LA, Salovaara R, Kristo P, et al: Incidence of hereditary nonpolyposis colorectal cancer and the feasibility of molecular screening for the disease. N Engl J Med 338:1481- 1487, 1998.
5. Chadwick RB, Pyatt RE, Niemann TH, et al: Hereditary and somatic DNA mismatch repair gene mutations in sporadic endometrial carcinoma. J Med Genet 38:461-466, 2001.
6. Banno K, Susumu N, Yanokura M, et al: Association of HNPCC and endometrial cancers. Int J Clin Oncol 9:262-269, 2004.
7. Dunlop MG, Farrington SM, Carothers AD, et al: Cancer risk associated with germline DNA mismatch repair gene mutations. Hum Mol Genet 6:105-110, 1997.
8. Liu B, Parsons R, Papadopoulos N, et al: Analysis of mismatch repair genes in hereditary non-polyposis colorectal cancer patients. Nat Med 2:169-174, 1996.
9. Nystrom-Lahti M, Wu Y, Moisio AL, et al: DNA mismatch repair gene mutations in 55 kindreds with verified or putative hereditary non-polyposis colorectal cancer. Hum Mol Genet 5:763-769, 1996.
10. Lengauer C, Kinzler KW, Vogelstein B: Genetic instabilities in human cancers. Nature 396:643-649, 1998.
11. Loeb LA: Mutator phenotype may be required for multistage carcinogenesis. Cancer Res 51:3075-3079, 1991.
12. Parsons R, Li GM, Longley MJ, et al: Hypermutability and mismatch repair deficiency in RER+ tumor cells. Cell 75:1227- 1236, 1993.
13. Boland CR, Thibodeau SN, Hamilton SR, et al: A national cancer institute workshop on microsatellite instability for cancer detection and familial predisposition: Development of international criteria for the determination of microsatellite instability in colorectal cancer. Cancer Res 58:5248-5257, 1998.
14. Aarnio M, Sankila R, Pukkala E, et al: Cancer risk in mutation carriers of DNA-mismatch- repair genes. Int J Cancer 81:214-218, 1999.
15. Watson P, Lynch HT: Extracolonic cancer in hereditary nonpolyposis colorectal cancer. Cancer 71:677-685, 1993.
16. Watson P, Vasen HF, Mecklin JP, et al: The risk of endometrial cancer in hereditary nonpolyposis colorectal cancer. Am J Med 96:516-520, 1994.
17. Vasen HFA, Johan G, Offerhaus A, et al: The tumour spectrum in hereditary non-polyposis colorectal cancer: A study of 24 kindreds in the Netherlands. Int J Cancer 46:31-34, 1990.
18. Li GM and Modrich P: Restoration of mismatch repair to nuclear extracts of H6 colorectal tumor cells by a heterodimer of human MutL homologs. Proc Natl Acad Sci U S A 92:1950-1954, 1995.
19. de Wind N, Dekker M, Berns A, et al: Inactivation of the mouse Msh2 gene results in mismatch repair deficiency, methylation tolerance, hyperrecombination, and predisposition to cancer. Cell 82:321-330, 1995.
20. Edelmann W, Yang K, Umar A, et al: Mutation in the mismatch repair gene Msh6 causes cancer susceptibility. Cell 91:467-477, 1997.
21. Jiricny J: Mediating mismatch repair. Nat Genet 24:6-8, 2000.
22. Gurin CC, Federici MG, Kang L, et al: Causes and consequences of microsatellite instability in endometrial carcinoma. Cancer Res 59:462-466, 1999.
23. Cohn DE, Basil JB, Venegoni AR, et al: Absence of PTEN repeat tract mutation in endometrial cancers with microsatellite instability. Gynecol Oncol 79:101-106, 2000.
24. Vassileva V, Millar A, Briollais L, et al: Apoptotic and growth regulatory genes as mutational targets in mismatch repair deficient endometrioid adenocarcinomas of young patients. Oncol Rep 11:931-937, 2004.
25. Zhou XP, Kuismanen S, Nystrom-Lahti M, et al: Distinct PTEN mutational spectra in hereditary non-polyposis colon cancer syndrome- related endometrial carcinomas compared to sporadic microsatellite unstable tumors. Hum Mol Genet 11:445-450, 2002.
26. Vassileva V, Millar A, Briollais L, et al: Genes involved in DNA repair are mutational targets in endometrial cancers with microsatellite instability. Cancer Res 62:4095- 4099, 2002.
27. Fishel R: The selection for mismatch repair defects in hereditary nonpolyposis colorectal cancer: Revising the mutator hypoth esis. Cancer Res 61:7369-7374, 2001.
28. Zhang H, Richards B, Wilson T, et al: Apoptosis induced by overexpression of hMSH2 or hMLH1. Cancer Res 59:3021-3027, 1999.
29. Katabuchi H, van Rees B, Lambers AR, et al: Mutations in DNA mismatch repair genes are not responsible for microsatellite instability in most sporadic endometrial carcinomas. Cancer Res 55:5556-5560, 1995.
30. Kobayashi K, Matsushima M, Koi S, et al: Mutational analysis of mismatch repair genes, hMLH1 and hMSH2, in sporadic endometrial carcinomas with microsatellite instability. Jpn J Cancer Res 87:141-145, 1996.
31. Lim PC, Tester D, Cliby W, et al: Absence of mutations in DNA mismatch repair genes in sporadic endometrial tumors with microsatellite instability. Clin Cancer Res 2:1907-1911, 1996.
32. Kowalski LD, Mutch DG, Herzog TJ, et al: Mutational analysis of MLH1 and MSH2 in 25 prospectively-acquired RER+ endometrial cancers. Genes Chromosomes Cancer 18:219- 227, 1997.
33. Esteller M, Levine R, Baylin SB, et al: MLH1 promoter hypermethylation is associated with the microsatellite instability phenotype in sporadic endometrial carcinomas. Oncogene 16:2413-2417, 1998.
34. Goodfellow PJ, Buttin BM, Herzog TJ, et al: Prevalence of defective DNA mismatch repair and MSH6 mutation in an unselected series of endometrial cancers. Proc Natl Acad Sci U S A 100:5908-5913, 2003.
35. Simpkins SB, Bocker T, Swisher EM, et al: MLH1 promoter methylation and gene silencing is the primary cause of microsatellite instability in sporadic endometrial cancers. Hum Mol Genet 8:661-666, 1999.
36. Sumoi R, Hakala-Ala-Pietila T, Leminen A, et al: Hereditary aspects of endometrial adenocarcinoma. Int J Cancer 62:132-137, 1995.
37. Wijnen J, De Leeuw W, Vasen H, et al: Familial endometrial cancer in female carriers of MSH6 germline mutations. Nat Genet 23:142-144, 1999.
38. Miyaki M, Konishi M, Tanaka K, et al: Germline mutation of MSH6 as the cause of hereditary nonpolyposis colorectal cancer. Nat Genet 17:271-272, 1997.
39. Hendriks YM, Wagner A, Morreau H, et al: Cancer risk in hereditary nonpolyposis colorectal cancer due to MSH6 mutations: impact on counseling and surveillance. Gastroenterology 127:17-25, 2004.
40. Gruber SB, Thompson WD: A population- based study of endometrial cancer and familial risk in younger women: Cancer and Steroid Hormone Study Group. Cancer Epidemiol Biomarkers Prev 5:411-417, 1996.
41. Millar AL, Pal T, Madlensky L, et al: Mismatch repair gene defects contribute to the genetic basis of double primary cancers of the colorectum and endometrium. Hum Mol Genet 9:823-829, 1999.
42. Whelan A, Babb S, Mutch D, et al: MSI in endometrial carcinoma: Absence of MLH1 promoter methylation is associated with increased familial risk for cancers. Int J Cancer 99:697-704, 2002.
43. Pal T, Flanders T, Mitchell-Lehman M, et al: Genetic implications of double primary cancers of the colorectum and endometrium. J Med Genet 35:978-984, 1998.
44. Buttin BM, Powell MA, Mutch DG, et al: Increased risk for HNPCC-associated synchronous and metachronous malignancies in patients with MSI-positive endometrial carcinoma lacking MLH1 promoter methylation. Clin Cancer Res 10:481-490, 2004.
45. Jarvinen HJ, Aarnio M, Mustonen H, et al: Controlled 15-year trial on screening for colorectal cancer in families with hereditary nonpolyposis colorectal cancer. Gastroenterology 118:829-834, 2000.
46. Boks DE, Trujillo AP, Voogd AC, et al: Survival analysis of endometrial carcinoma associated with hereditary nonpolyposis colorectal cancer. Int J Cancer 102:198-200, 2002.
47. Caduff RF, Johnston CM, Svoboda- Newman SM, et al: Clinical and pathological significance of microsatellite instability in sporadic endometrial carcinoma. Am J Pathol 148:1671-1678, 1996.
48. MacDonald ND, Salvesen HB, Ryan A, et al: Frequency and prognostic impact of microsatellite instability in a large populationbased study of endometrial carcinomas. Cancer Res 60:1750-1752, 2000.
49. Maxwell GL, Risinger JI, Alvarez AA, et al: Favorable survival associated with microsatellite instability in endometrioid endometrial cancers. Obstet Gynecol 97:417-422, 2001.
50. Basil JB, Goodfellow PJ, Rader JS, et al: Clinical significance of microsatellite instability in endometrial carcinoma. Cancer 89:1758-1764, 2000.
51. Risch HA, McLaughlin JR, Cole DE, et al: Prevalence and penetrance of germline BRCA1 and BRCA2 mutations in a population series of 649 women with ovarian cancer. Am J Hum Genet 68:700-710, 2001.
52. Orth K, Hung J, Gazdar A, et al: Genetic instability in human ovarian cancer cell lines. Proc Natl Acad Sci U S A 91:9495-9499, 1994.
53. Geisler JP, Goodheart MJ, Sood AK, et al: Mismatch repair gene expression defects contribute to microsatellite instability in ovarian carcinoma. Cancer 98:2199-2206, 2003.
54. Stratton JF, Thompson D, Bobrow L, et al: The genetic epidemiology of early-onset epithelial ovarian cancer: A population-based study. Am J Hum Genet 65:1725-1732, 1999.
55. Shannon C, Kirk J, Barnetson R, et al: Incidence of microsatellite instability in synchronous tumors of the ovary and endometrium. Clin Cancer Res 9:1387-1392, 2003.
56. Arzimanoglou, II, Lallas T, Osborne M, et al: Microsatellite instability differences between familial and sporadic ovarian cancers. Carcinogenesis 17:1799-1804, 1996.
57. Rubin SC, Benjamin I, Behbakht K, et al: Clinical and pathological features of ovarian cancer in women with germline mutations of BRCA1. N Engl J Med 335:1413-1416, 1996.
58. Watson P, Butzow R, Lynch HT, et al: The clinical features of ovarian cancer in hereditary nonpolyposis colorectal cancer. Gynecol Oncol 82:223-228, 2001.
59. Brown R, Hirst GL, Gallagher WM, et al: hMLH1 expression and cellular responses of ovarian tumour cells to treatment with cytotoxic anticancer agents. Oncogene 15:45-52, 1997.
60. Strathdee G, MacKean MJ, Illand M, et al: A role for methylation of the hMLH1 promoter in loss of hMLH1 expression and drug resistance in ovarian cancer. Oncogene 18:2335-2341, 1999.
61. Gifford G, Paul J, Vasey PA, et al: The acquisition of hMLH1 methylation in plasma DNA after chemotherapy predicts poor survival for ovarian cancer patients. Clin Cancer Res 10:4420-4426, 2004.
62. Levin B, Ludwig KA, Steinbach G, for the National Comprehensive Cancer Network Colorectal Screening Panel: Colorectal Screening: Practice Guidelines in Oncology, v. 1.2005.
63. Risinger JI, Berchuck A, Kohler MF, et al: Genetic instability of microsatellites in endometrial carcinoma. Cancer Res 53:5100- 5103, 1993.
64. Berends MJ, Hollema H, Wu Y, et al: MLH1 and MSH2 protein expression as a prescreening marker in hereditary and non-hereditary endometrial hyperplasia and cancer. Int J Cancer 92:398-403, 2001.
65. Berends MJ, Wu Y, Sijmons RH, et al: Toward new strategies to select young endometrial cancer patients for mismatch repair gene mutation analysis. J Clin Oncol 21:4364-4370, 2003.
66. Halbert CH, Lynch H, Lynch J, et al: Colon cancer screening practices following genetic testing for hereditary nonpolyposis colon cancer (HNPCC) mutations. Arch Intern Med 164:1881-1887, 2004.
67. Hadley DW, Jenkins JF, Dimond E, et al: Colon cancer screening practices after genetic counseling and testing for hereditary nonpolyposis colorectal cancer. J Clin Oncol 22:39-44, 2004.
68. Hall MA, Rich SS: Laws restricting health insurers' use of genetic information: Impact on genetic discrimination. Am J Hum Genet 66:293-307, 2000.
69. Stephenson J: Genetic test information fears unfounded. JAMA 282:2197-2198, 1999.
70. Dove-Edwin I, Boks D, Goff S, et al: The outcome of endometrial carcinoma surveillance by ultrasound scan in women at risk of hereditary nonpolyposis colorectal carcinoma and familial colorectal carcinoma. Cancer 94:1708-1712, 2002.
71. Rijcken FE, Mourits MJ, Kleibeuker JH, et al: Gynecologic screening in hereditary nonpolyposis colorectal cancer. Gynecol Oncol 91:74-80, 2003.
72. Syngal S, Weeks JC, Schrag D, et al: Benefits of colonoscopic surveillance and prophylactic colectomy in patients with hereditary nonpolyposis colorectal cancer mutations. Ann Intern Med 129:787-796, 1998.
73. Annie Yu HJ, Lin KM, Ota DM, et al: Hereditary nonpolyposis colorectal cancer: Preventive management. Cancer Treat Rev 29:461-470, 2003.
74. Ruschoff J, Wallinger S, Dietmaier W, et al: Aspirin suppresses the mutator phenotype associated with hereditary nonpolyposis colorectal cancer by genetic selection. Proc Natl Acad Sci U S A 95:11301-11306, 1998.
75. Lu K: Endometrial cancer in women with HNPCC. Future challenges in clinical and translational research for endometrial cancer (abstract). Int J Gynecol Cancer 15(2):400-401, 2005.
76. Syngal S, Fox EA, Eng C, et al: Sensitivity and specificity of clinical criteria for hereditary non-polyposis colorectal cancer associated mutations in MSH2 and MLH1. J Med Genet 37:641-645, 2000.
77. Burke W, Petersen G, Lynch P, et al: Recommendations for follow-up care of individuals with an inherited predisposition to cancer. I. Hereditary nonpolyposis colon cancer. Cancer Genetics Studies Consortium. JAMA 277:915-919, 1997.
78. Vasen HF, Mecklin JP, Khan PM, et al: The International Collaborative Group on Hereditary Non-Polyposis Colorectal Cancer (ICGHNPCC). Dis Colon Rectum 34:424-425, 1991.
79. Bellacosa A, Genuardi M, Anti M, et al: Hereditary nonpolyposis colorectal cancer: Review of clinical, molecular genetics, and counseling aspects. Am J Med Genet 62:353-364, 1996.
80. Vasen HFA, Watson P, Mecklin J-P, et al: New clinical criteria for hereditary nonpolyposis colorectal cancer (HNPCC, Lynch syndrome) proposed by the International Collaborative Group on HNPCC. Gastroenterology 116:1453-1456, 1999.
81. Rodriguez-Bigas MA, Boland CR, Hamilton SR, et al: A national cancer institute workshop on hereditary nonpolyposis colorectal cancer syndrome: meeting highlights and Bethesda guidelines. J Natl Cancer Inst 89:1758-1761, 1997.