Surgical Staging in Endometrial Cancer
Early presentation of endometrial cancer permits effective managementwith excellent clinical outcome. The addition of hysteroscopy todilatation and curettage (D&C) in the evaluation of postmenopausalbleeding adds little to the detection of malignancy. Imaging studies suchas computed tomography, magnetic resonance imaging, and positronemissiontomography may be of use in determining the presence ofextrauterine disease in patients medically unfit for surgical staging.However, these studies are not sufficiently sensitive to replace surgicalstaging and have little role in routine preoperative evaluation. Clinicalstaging alone is clearly inadequate, as 23% of preoperative clinicalstage I/II patients are upstaged with comprehensive surgical staging.Preoperative tumor grade from D&C or office biopsy may be inaccurateand lead to an underestimate of tumor progression if used to determinewhich patients should be surgically staged. Clinical estimationof depth of invasion, with or without frozen section, is inaccurate andmay lead to underestimation of disease status when surgical staging isnot performed. The practice of resecting only clinically suspicious nodesshould be discouraged as it is no substitute for comprehensive surgicalstaging. Comprehensive surgical staging provides proper guidance forpostoperative adjuvant therapy, avoiding needless radiation in 85% ofclinical stage I/II patients. Finally, resection of occult metastasis withsurgical staging may improve survival.
Early presentation of endometrial cancer permits effective management with excellent clinical outcome. The addition of hysteroscopy to dilatation and curettage (D&C) in the evaluation of postmenopausal bleeding adds little to the detection of malignancy. Imaging studies such as computed tomography, magnetic resonance imaging, and positronemission tomography may be of use in determining the presence of extrauterine disease in patients medically unfit for surgical staging. However, these studies are not sufficiently sensitive to replace surgical staging and have little role in routine preoperative evaluation. Clinical staging alone is clearly inadequate, as 23% of preoperative clinical stage I/II patients are upstaged with comprehensive surgical staging. Preoperative tumor grade from D&C or office biopsy may be inaccurate and lead to an underestimate of tumor progression if used to determine which patients should be surgically staged. Clinical estimation of depth of invasion, with or without frozen section, is inaccurate and may lead to underestimation of disease status when surgical staging is not performed. The practice of resecting only clinically suspicious nodes should be discouraged as it is no substitute for comprehensive surgical staging. Comprehensive surgical staging provides proper guidance for postoperative adjuvant therapy, avoiding needless radiation in 85% of clinical stage I/II patients. Finally, resection of occult metastasis with surgical staging may improve survival.
Endometrial cancer is the most common gynecologic malignancy in the United States, with nearly 40,000 cases reported annually (approximately 1 in 37 American women).[1] Fortunately, most women present with the onset of symptoms, namely abnormal uterine bleeding or discharge, when disease is limited to the uterine corpus. This early presentation of disease allows for effective management with excellent clinical outcome, leading to only 7,300 deaths per year. Overall 5-year survival for patients with surgical stage I disease is reported at 85% or higher.[2,3] Recently, endometrial cancer has been categorized into two distinct clinical types. Type I tumors include the more classic endometrial malignancies associated with unopposed estrogenic stimulation of the endometrium from either pharmacologic or physiologic sources. Histologically these lesions are endometrioid in appearance and are clinically associated with obesity, hyperlipidemia, and endometrial hyperplasias. Most type I tumors are early-stage, low-grade tumors and are associated with an excellent prognosis. Type II tumors tend to be more aggressive, both clinically and in histologic appearance. They are associated with high-risk cell types including clear cell, uterine papillary serous carcinoma, as well as high-grade endometrioid tumors. Type II tumors tend to occur in thinner, older patients and are typically not hormonally responsive. Continued controversy surrounds the management of patients thought to have early-stage tumors limited to the uterine corpus (International Federation of Gynecology and Obstetrics [FIGO] stage I). Specifically, the role of comprehensive surgical staging, including pelvic and para-aortic lymphadenectomy for all patients, has been questioned. Strategies utilizing pre- or postoperative histologic grade and depth of invasion by frozen section or gross inspection have been advocated by some to select only higher-risk patients for complete surgical staging. Cost, survival, and the utilization of adjuvant therapies are also important issues in the management of patients with endometrial cancer.
Diagnosis and Preoperative Evaluation Most patients with endometrial cancer present with abnormal uterine bleeding or postmenopausal bleeding leading to subsequent evaluation. An endometrial biopsy, D&C, and/or vaginal probe ultrasound may be performed. Should a diagnosis of atypical hyperplasia be reported, the clinician should be aware that up to 40% of patients with atypical hyperplasias on biopsy or D&C have evidence of an adenocarcinoma on final hysterectomy pathology.[4] Additionally, these tumors are not always earlystage, low-grade tumors. As many as 31% of these patients will have advanced- grade tumors or evidence of myometrial invasion on final pathology.[ 4] Therefore, it is imperative that these patients be managed by physicians capable of performing comprehensive surgical staging in the event that cancer is found at the time of surgery. Hysteroscopy
Hysteroscopy has been advocated as an adjunct to D&C. Unguided D&C may have a false-negative rate of 10% to 30% in the evaluation of postmenopausal bleeding.[5] Unfortunately, hysteroscopy combined with D&C may also have false-negative rates of up to 20%. Concern remains that the routine use of hysteroscopy may increase the rate of positive cytology at the time of surgical staging.[6] Therefore, the addition of hysteroscopy to D&C in the evaluation of postmenopausal bleeding seems to add little to current management. Preoperative Imaging
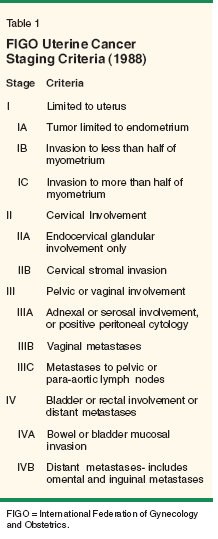
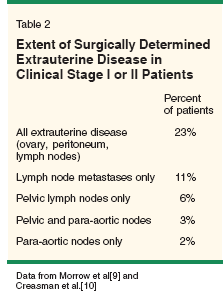
The initial diagnostic exam should include a complete physical examination, with particular attention paid to possible metastatic sites such as peripheral lymph nodes (supraclavicular, inguinal), the presence of abdominal masses or ascites, vaginal metastases or gross cervical involvement, uterine size and/or parametrial involvement. The role of preoperative imaging in the evaluation of endometrial cancer, particularly as it relates to diagnosing metastatic disease in clinical stage I/II tumors, remains less clear. A preoperative chest x-ray is noted to be abnormal in 2% of women with endometrial cancer and may serve to diagnose concomitant comorbidities. While other imaging technologies including computed tomography (CT) or magnetic resonance imaging (MRI) have been used to predict depth of myometrial involvement, these techniques appear to have limited utility in accurately detecting the presence of extrauterine disease. False-positive rates of 10% and false-negative rates of 8% to 35% have been reported.[7] The addition of positron-emission tomography (PET) scanning to CT has proven to be only 60% sensitive with 94% to 98% specificity in accurately detecting extrauterine disease.[8] Therefore, these imaging techniques (CT, MRI, PET) may be more suited for detecting extrauterine disease in patients who are medically unfit for comprehensive surgical staging and not as a replacement for proper surgical assessment of metastatic disease. FIGO Staging The surgical staging system as established by FIGO in 1988 is shown in Table 1. Clinical staging for endometrial cancer has largely been abandoned in favor of surgical staging, as clinical staging fails to take into account histopathologic features that more accurately delineate patients who may benefit from adjuvant therapy. Such features include tumor grade, depth of invasion, histologic subtype, lymphovascular space invasion, and nodal metastases.[9] Clinical staging alone is inadequate, as 23% of preoperative clinical stage I/II patients will be upstaged with extensive surgical staging (Table 2).[10] Staging Procedure
The surgical staging procedure for patients with endometrial cancer should include an examination under adequate anesthesia, followed by adequate surgical exposure and inspection of intraabdominal structures with biopsy of any suspicious lesions. Lavage peritoneal cytology should be obtained prior to manipulation of the uterus. A complete extrafascial hysterectomy with bilateral salpingo-oophorectomy should be performed. Pelvic and para-aortic retroperitoneal lymph node dissection should be performed. The boundaries of the lymphadenectomy should include the genitofemoral nerve laterally, the hypogastric artery medially, the obturator nerve posteriorly, the circumflex iliac vein inferiorly, and the origin of the inferior mesenteric artery (some claim the superior mesenteric artery) superiorly, as described per the standardized Gynecologic Oncology Group (GOG) protocol.[9]
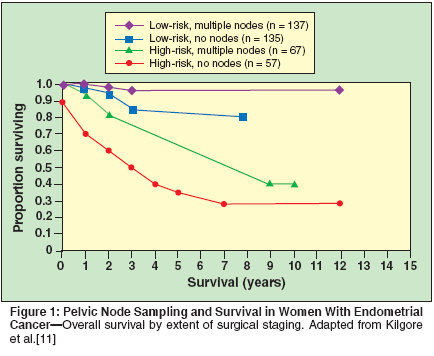
The appropriate extent of the retroperitoneal lymph node dissection is debated by some, although most agree that numerous sites should be assessed. The argument for complete lymphadenectomy during the staging procedure has its basis in statistical modeling for the detection of positive nodes. In order to have an 80% chance of detecting a single node that is positive (if only 5% of nodes are positive at that particular site) requires that at least 50% of that site's nodes be sampled. Additionally, previous studies have shown that if pelvic nodes are positive, 40% to 50% of patients have para-aortic nodal involvement. The role of lymph node dissection has been validated in clinical studies, as patients undergoing extended nodal dissection (four or more sites) had a better survival than those who did not undergo nodal sampling (Figure 1).[11] This survival advantage held true for the entire population (P < .001), highrisk patients only (P < .001), and highrisk patients treated with adjuvant radiotherapy (P = .01). These findings were confirmed in a recent publication by Lutman et al, who reported that high-risk subtype patients with at least 11 lymph nodes evaluated had significantly improved survival.[12] In addition to the above noted surgical approach, in high-risk patients such as those with clear cell or uterine papillary serous carcinoma, infracolic omentectomy should be considered, as these histologic types may be associated with omental metastases similar to ovarian carcinoma.[13] Intraoperative Staging Decisions
The decision to perform comprehensive surgical staging for patients with endometrial adenocarcinoma should ideally be made prior to surgery. However, some advocates recommend making this decision in the operating room based on a combination of preoperative grade and histology, intraoperative assessment of the presence and depth of myometrial invasion either grossly or with frozen section, and clinical assessment of nodal spread intraoperatively. Preoperative D&C or biopsy tumor grade is not sufficient to determine which patients should be surgically staged. Daniel et al reported 15% to 20% of cases had their tumor grade upgraded on final pathology, with only a 57% to 68% correlation of tumor grade between D&C and final pathology.[14] In addition, final cell type is not well correlated with D&C. In a study of biopsy-proven clinical stage I, grade 1, endometrioid tumors (typically low-risk), 19% were upgraded to a higher grade or had a change in preoperative histology compared to final histology.[15] Specifically, 15% of patients were upgraded to grade 2 tumors, 0.5% to grade 3, 2.5% to a serous or clear cell histology, and 1% to a carcinosarcoma histology. Grade and histology migration correspond to an increased risk of nodal metastases and may potentiate the need for adjuvant radiation therapy if the patient is not surgically staged. Others argue that intraoperative algorithms be used to determine which patients need surgical staging. These algorithms depend on clinical estimation of depth of invasion (DOI) combined with the aforementioned preoperative grade. However, gross estimation of depth of invasion becomes less accurate as tumor grade increases.[16] For grade 1 tumors (final pathology grade), clinical estimation of DOI is 87% accurate, whereas for grade 3 tumors, such estimates are only 30% accurate. Using frozen section to improve intraoperative grade or DOI estimation may not be helpful, as frozen section was not shown to be fully predictive of grade (84% accuracy) or myometrial invasion (88% accuracy).[17] Additionally, combining clinical estimation of DOI with frozen section or preoperative grade was not predictive of final surgical stage.[18] The practice of resecting only clinically suspicious nodes is also insufficient, as 36% of positive lymph nodes are missed by palpation.[19] Nearly 50% of positive nodes are < 1 cm,[20] and less than 30% of positive nodes are palpably abnormal.[21]
Most advocates of preoperative or intraoperative algorithms to determine which patients should be surgically staged refer to the potential for increased morbidity with the staging procedure. However, multiple authors have found no difference in morbidity (8%) associated with the staging procedure and simple abdominal hysterectomy in this higher-risk and often morbidly obese population.[22,23] Prospective data have demonstrated that the median time for lymphadenectomy is only 24 minutes, with less than 25 mL median blood loss attributed to the lymphadenectomy portion of the procedure.[24] Additionally, the average hospital stay for patients undergoing comprehensive surgical staging in conjunction with their hysterectomy is less than 4 days.[25] Benefits of Surgical Staging
The demonstration that no disease exists outside the uterus allows one to observe patients otherwise at risk for nodal metastases and recurrence without the use of potentially morbid adjuvant radiation therapy. When patients are managed without complete surgical staging information the clinician may be forced to prescribe adjuvant therapy based merely on clinical assumption and potential risk. Accordingly, nonjudicious use of adjuvant therapy may increase morbidity and cost of care without a proven benefit. Individual and combined evidence from two prospective randomized studies involving over 1,200 patients failed to demonstrate a survival benefit when pelvic irradiation was administered to the unstaged patient, regardless of the presence of specific uterine risk factors.[3,26] Thus, the administration of postoperative teletherapy in unstaged patients may subject these women to ineffective treatment and a 3% to 7% risk of severe and 20% risk of mild radiation- associated complications.[27-29]
- Postoperative Radiation vs Observation- Recently, the GOG reported final data from a randomized trial of adjuvant radiation therapy for patients with intermediate-risk endometrial cancer following complete surgical staging.[30] Patients with surgical stage IB, IC and occult stage II endometrial carcinoma were randomized to observation or whole-pelvic radiation therapy postoperatively. The study demonstrated that adjuvant radiation therapy decreased pelvic recurrence (12% vs 3%, P = .007), but at the cost of increased complications with no improvement in overall survival (86% vs 92%, P = .557). Therefore, a strategy maximizing surgical staging while minimizing the use of adjuvant radiation therapy may decrease overall morbidity and cost, at the expense of an increase in the incidence of vaginal metastases.
Retrospective data[2,23] support an observation-only strategy in intermediate risk patients, which includes all grade 3 tumors, stage IB grade 2 or 3 tumors, and all stage IC patients when surgically staged. Recent studies support this approach for endometrial cancer patients, documenting a salvage rate for vaginal recurrences of 63% and no difference in overall survival when compared to patients receiving adjuvant radiation therapy.[30,31]
- Further Support for Surgical Staging-Routine performance of comprehensive surgical staging is cost-effective and may result in a 31% decrease in costs compared to intraoperative decision algorithms.[31,32] In recognition of the importance of surgical staging, GOG Protocol 210, a prospective study with the goal of developing a molecular disease classification system to complement FIGO staging, now requires full surgical staging including para-aortic and high para-aortic lymphadenectomy. Additionally, the American College of Obstetricians and Gynecologists (ACOG) endorsed the importance of surgical staging in a practice bulletin issued September 15, 2004, stating:
Every patient undergoing surgery for the treatment of endometrial cancer should be counseled preoperatively as to the possible need and benefit of staging and should be offered the option at the time of their initial surgical procedure.
- Role of Gynecologic Oncologist- The preferred strategy to employ comprehensive surgical staging for all patients with endometrial cancer requires all patients to undergo surgery conducted by a gynecologic oncologist (or gynecologist with general surgery backup). Unfortunately, only 32% of women with endometrial cancer in the United States currently have surgery performed by a gynecologic oncologist. An additional 11% have a gynecologic oncologist on standby.[ 33]
In a patterns-of-care study, Roland et al reported only a 26% histologically confirmed lymph node assessment in patients operated by a non-gynecologic oncologist compared to 83% of patients when surgery was performed by a gynecologic oncologist.[34] Additionally, this study demonstrated that complete tumor- node-metastasis (TNM) staging was successfully performed by gynecologic oncologists 94% of the time, as compared to 45.2% by non-gynecologic oncologists. More importantly, only 6 patients (8.6%) with intermediate-risk disease deemed at risk for extrauterine spread received radiation when managed by gynecologic- oncologists vs 15 patients (21.7%) managed by non-gynecologic oncologists secondary to adequate surgical staging.
Laparoscopy in the Management of Endometrial Cancer Recently, laparoscopic surgery in the management of endometrial cancer has come to the forefront with the intent to reduce complications and recovery time in this difficult, obese, surgical population. Despite the difficulties of laparoscopy in these patients, studies have shown that the procedure is feasible 85% to 95% of the time.[35,36] The technique is similar to abdominal staging in that the abdomen is inspected, washings are obtained, and a complete para-aortic and pelvic lymphadenectomy is performed. Variations exist as to completion of the hysterectomy either vaginally or totally laparoscopically (the specimen may be extracted from the vagina after amputation). Several studies[35,37-41] have demonstrated safety in terms of postoperative complications, with some finding that complications were higher with open abdominal surgery. Longterm prospective outcomes, as well as safety data, are still pending from the prospective evaluation of exploratory laparotomy with staging vs laparoscopic hysterectomy and staging (GOG Protocol LAP2). However, retrospective studies have demonstrated no difference in survival.[39,40] One would expect that overall survival should be similar because most studies report at least equal (if not improved) nodal counts with laparoscopy compared to laparotomy. The purported benefits of the laparoscopic approach include an average 2-day shorter hospital stay. Despite an initial increase in hospital charges secondary to laparoscopy costs,[36] cost savings may be realized, as out-ofhospital expenses such as wound care, income loss, and lack of productivity in society tend to favor laparoscopy. Conclusions In conclusion, comprehensive surgical staging for endometrial cancer clearly is more advantageous than clinical staging. Surgical staging allows for determination of disease extent and detection and/or resection of occult metastases. Staging can be safely performed at the time of hysterectomy without added morbidity, and provides proper guidance with respect to postoperative adjuvant therapy, avoiding needless radiation therapy in 85% of clinical stage I/II patients. Finally, it appears to be the most costeffective strategy in this setting.
Disclosures:
The authors have no significant financial interest or other relationship with the manufacturers of any products or providers of any service mentioned in this article.
References:
1. Jemal A, Murray T, Ward E, et al: Cancer statistics, 2005. CA Cancer J Clin 55:10-30, 2005.
2. Straughn JM Jr, Huh WK, Kelly FJ, et al: Conservative management of stage I endometrial carcinoma after surgical staging. Gynecol Oncol 84:194-200, 2002.
3. Creutzberg CL, van Putten WL, Koper PC, et al: Surgery and postoperative radiotherapy versus surgery alone for patients with stage-1 endometrial carcinoma: Multicentre randomised trial. PORTEC Study Group. Post Operative Radiation Therapy in Endometrial Carcinoma. Lancet 355:1404-1411, 2000.
4. Trimble CL, Kauderer J, Silverberg S, et al Concurrent endometrial carcinoma in women with biopsy diagnosis of atypical endometrial hyperplasia: A GOG study, in Proceedings of the 35th Annual Meeting of Society of Gynecologic Oncologists, San Diego, Calif, 2004.
5. Zorlu CG, Cobanoglu O, Isik AZ, et al: Accuracy of pipelle endometrial sampling in endometrial carcinoma. Gynecol Obstet Invest 38:272-275, 1994.
6. Bradley WH, Boente MP, Brooker D, et al: Hysteroscopy and cytology in endometrial cancer. Obstet Gynecol 104:1030-1033, 2004.
7. Kinkel K, Kaji Y, Yu KK, et al: Radiologic staging in patients with endometrial cancer: A meta-analysis. Radiology 212:711-718, 1999.
8. Horowitz NS, Dehdashti F, Herzog TJ, et al: Prospective evaluation of FDG-PET for detecting pelvic and para-aortic lymph node metastasis in uterine corpus cancer. Gynecol Oncol 95:546-551, 2004.
9. Morrow CP, Bundy BN, Kurman RJ, et al: Relationship between surgical-pathological risk factors and outcome in clinical stage I and II carcinoma of the endometrium: A Gynecologic Oncology Group study. Gynecol Oncol 40:55-65, 1991.
10. Creasman WT, Morrow CP, Bundy BN, et al: Surgical pathologic spread patterns of endometrial cancer. A Gynecologic Oncology Group study. Cancer 60:2035-2041, 1987.
11. Kilgore LC, Partridge EE, Alvarez RD, et al: Adenocarcinoma of the endometrium: Survival comparisons of patients with and without pelvic node sampling. Gynecol Oncol 56:29-33, 1995.
12. Lutman CV, Cragun J, Havrilesky L, et al: Evaluation of pelvic lymph node count (PLNC) as a prognostic factor in FIGO stage I and II endometrial carcinoma, in Proceedings of the 36th Annual Meeting of the Society of Gynecologic Oncologists, Miami, Fla, 2005.
13. Geisler JP, Geisler HE, Melton ME, et al: What staging surgery should be performed on patients with uterine papillary serous carcinoma? Gynecol Oncol 74:465-467, 1999.
14. Daniel AG, Peters WA 3rd: Accuracy of office and operating room curettage in the grading of endometrial carcinoma. Obstet Gynecol 71:612-614, 1988.
15. Ben-Shachar I, Pavelka J, Cohn DE, et al: Surgical staging for patients presenting with grade 1 endometrial carcinoma. Obstet Gynecol 105:487-493, 2005.
16. Goff BA, Rice LW: Assessment of depth of myometrial invasion in endometrial adenocarcinoma. Gynecol Oncol 38:46-48, 1990.
17. Kucera E, Kainz C, Reinthaller A, et al: Accuracy of intraoperative frozen-section diagnosis in stage I endometrial adenocarcinoma. Gynecol Obstet Invest 49:62-66, 2000.
18. Frumovitz M, Singh DK, Meyer L, et al: Predictors of final histology in patients with endometrial cancer. Gynecol Oncol 95:463- 468, 2004.
19. Arango HA, Hoffman MS, Roberts WS, et al: Accuracy of lymph node palpation to determine need for lymphadenectomy in gynecologic malignancies. Obstet Gynecol 95:553-556, 2000.
20. Girardi F, Petru E, Heydarfadai M, et al: Pelvic lymphadenectomy in the surgical treatment of endometrial cancer. Gynecol Oncol 49:177-180, 1993.
21. Chuang L, Burke TW, Tornos C, et al: Staging laparotomy for endometrial carcinoma: Assessment of retroperitoneal lymph nodes. Gynecol Oncol 58:189-193, 1995.
22. Orr JW Jr, Holimon JL, Orr PF: Stage I corpus cancer: Is teletherapy necessary? Am J Obstet Gynecol 176:777-789 (incl discussion), 1997.
23. Ayhan A, Taskiran C, Celik C, et al: Is there a survival benefit to adjuvant radiotherapy in high-risk surgical stage I endometrial cancer? Gynecol Oncol 86:259-263, 2002.
24. Fanning J, Firestein S: Prospective evaluation of the morbidity of complete lymphadenectomy in endometrial cancer. Int J Gynecol Cancer 8:270-273, 1998.
25. Kennedy AW, Austin JM Jr, Look KY, et al: The Society of Gynecologic Oncologists Outcomes Task Force. Study of endometrial cancer: Initial experiences. Gynecol Oncol 79:379-398, 2000.
26. Aalders J, Abeler V, Kolstad P, et al: Postoperative external irradiation and prognostic parameters in stage I endometrial carcinoma: Clinical and histopathologic study of 540 patients. Obstet Gynecol 56:419-427, 1980.
27. Corn BW, Lanciano RM, Greven KM, et al: Impact of improved irradiation technique, age, and lymph node sampling on the severe complication rate of surgically staged endometrial cancer patients: A multivariate analysis. J Clin Oncol 12:510-515, 1994.
28. Creutzberg CL, van Putten WL, Koper PC, et al: The morbidity of treatment for patients with stage I endometrial cancer: Results from a randomized trial. Int J Radiat Oncol Biol Phys 51:1246-1255, 2001.
29. Greven KM, Lanciano RM, Herbert SH, et al: Analysis of complications in patients with endometrial carcinoma receiving adjuvant irradiation. Int J Radiat Oncol Biol Phys 21:919- 923, 1991.
30. Keys HM, Roberts JA, Brunetto VL, et al: A phase III trial of surgery with or without adjunctive external pelvic radiation therapy in intermediate risk endometrial adenocarcinoma: A Gynecologic Oncology Group study. Gynecol Oncol 92:744-751, 2004.
31. Fanning J, Hoffman ML, Andrews SJ, et al: Cost-effectiveness analysis of the treatment for intermediate risk endometrial cancer: Postoperative brachytherapy vs. observation. Gynecol Oncol 93:632-636, 2004.
32. Barnes MN, Roland PY, Straughn M, et al: A comparison of treatment strategies for endometrial adenocarcinoma: Analysis of financial impact. Gynecol Oncol 74:443-447, 1999.
33. Partridge EE, Shingleton HM, Menck HR: The National Cancer Data Base report on endometrial cancer. J Surg Oncol 61:111-123, 1996.
34. Roland PY, Kelly FJ, Kulwicki CY, et al: The benefits of a gynecologic oncologist: A pattern of care study for endometrial cancer treatment. Gynecol Oncol 93:125-130, 2004.
35. Eltabbakh GH, Shamonki MI, Moody JM, et al: Laparoscopy as the primary modality for the treatment of women with endometrial carcinoma. Cancer 91:378-387, 2001.
36. Eltabbakh GH, Shamonki MI, Moody JM, et al: Hysterectomy for obese women with endometrial cancer: Laparoscopy or laparotomy? Gynecol Oncol 78:329-335, 2000.
37. Scribner DR Jr, Walker JL, Johnson GA, et al: Laparoscopic pelvic and paraaortic lymph node dissection: analysis of the first 100 cases. Gynecol Oncol 82:498-503, 2001.
38. Gemignani ML, Curtin JP, Zelmanovich J, et al: Laparoscopic-assisted vaginal hysterectomy for endometrial cancer: Clinical outcomes and hospital charges. Gynecol Oncol 73:5-11, 1999.
39. Holub Z, Jabor A, Bartos P, et al: Laparoscopic pelvic lymphadenectomy in the surgical treatment of endometrial cancer: Results of a multicenter study. JSLS 6:125-131, 2002.
40. Malur S, Possover M, Michels W, et al: Laparoscopic-assisted vaginal versus abdominal surgery in patients with endometrial cancer- a prospective randomized trial. Gynecol Oncol 80:239-244, 2001.
41. Kuoppala T, Tomas E, Heinonen PK: Clinical outcome and complications of laparoscopic surgery compared with traditional surgery in women with endometrial cancer. Arch Gynecol Obstet 270:25-30, 2004.