Hereditary Breast and Ovarian Cancer: High-Risk Management
The patient, DB, is a 51-year-old white, married female with a strong family history of breast cancer. She presented for high-risk assessment and genetic testing following the discovery of a deleterious mutation in a family member.
The patient, DB, is a 51-year-old white, married female with a strong family history of breast cancer. She presented for high-risk assessment and genetic testing following the discovery of a deleterious mutation in a family member.
TREATMENT SUMMARY
Initial high-risk evaluation was conducted in February 2004. DB's lifetime risk of breast cancer as calculated from the Gail model was 17.1%; by the Claus model, however, her risk was 34.3%. Her risk level qualified her to be followed in our high-risk program, in the "high-risk" group. This entailed our seeing the patient every 6 months and alternating mammogram and ultrasound with clinical breast exam (CBE) at one visit and magnetic resonance imaging (MRI) of the breast with CBE on the next visit, or ultrasound and CBE. (In the "high risk" group, MRI is performed every 2 years.)
DB's cousin had also initiated genetic testing, so we encouraged DB to contact us when her cousin's results were obtained, for appropriate follow-up. In March 2004, a deleterious mutation was discovered in her cousin, and DB presented for genetic counseling and testing to determine if she had the same mutation. A single-site analysis for the BRCA1 mutation previously documented in DB's cousin was performed on DB's blood; she was found to have the same mutation, thus making her risk of breast cancer approximately 87% in her lifetime.[1]
At the disclosure session, DB was informed that her genetic status made her eligible for the "very high risk" group in our high-risk program, instead of the "high risk" group. The difference between the two categories is in the frequency of MRI screening, which increases from every 2 years to every year in patients with the highest risk.
DB was concerned about insurance coverage for her MRI and was given the telephone number of the business office at our facility. She was also informed that she might qualify for a National Institutes of Health study that would cover the cost of MRI. DB was counseled about taking tamoxifen for chemoprevention, and about prophylactic mastectomy with reconstruction, and high-risk management of ovarian cancer. She was referred to a gynecologic oncologist to discuss prophylactic oophorectomy.
DB received a prophylactic oophorectomy in July 2004 and was seen in the high-risk breast clinic in August 2004. She mentioned that she had anxiety related to the out-of-pocket expenses for her prophylactic oophorectomy. She had not been successful in obtaining insurance approval for her MRI and wanted to wait until her next visit to undergo this procedure.
We therefore offered her an ultrasound and CBE in lieu of a screening MRI, and planned to perform an MRI as soon as possible, when her personal finances and/or insurance coverage made the procedure financially feasible for her. (Also, DB was mildly claustrophobic and was afraid to undergo the MRI. Oral valium was offered, but DB felt that delaying the MRI was the right decision for her.)
DB returned for her routine high-risk appointment in February 2005. She was due for a mammogram and wanted to undergo the mammogram, ultrasound, and CBE but schedule the MRI for a later date. No discrete or dominant masses were felt on the CBE, and the mammogram was read as benign.
On ultrasound, a 1.9-cm mass was detected on the inframammary ridge of the left breast. The location of the mass made it difficult to image with a mammogram and difficult to feel on CBE. The mass was biopsied and a moderately differentiated infiltrating ductal carcinoma was diagnosed as well as extensive ductal carcinoma in situ. A preoperative MRI was performed a few days later for surgical decision-making. On DB's MRI, the mass was easily visualized. DB underwent bilateral mastectomy and was determined to have stage II breast cancer.
NURSING MANAGEMENT
DB's initial high-risk assessment and genetic counseling for hereditary breast and ovarian cancer included a comprehensive evaluation of family history for potential hereditary syndromes and a review of personal history risk factors. This was conducted by an advanced practice nurse specializing in oncology, genetics, and breast health. DB was 50 years of age on her initial exam; she had started menarche at 13 years, had her first full-term pregnancy at age 27, and had one first-degree relative with breast cancer and one first-degree relative with ovarian cancer.
Using the Gail model, DB's risk was calculated at 17.1 lifetime risk.[2] The Claus model allows evaluation of risk based on second-degree relatives, which is often relevant when the hereditary risk may come from the paternal side, as was the case for DB.[3] It also allows for calculation of risk based on first-degree relatives with breast and ovarian cancer. Using the Claus table, DB's lifetime risk of breast cancer was calculated to be 34.3%.
An initial review of family history indicated that DB had multiple family members with early-onset breast cancer, ovarian cancer, and pancreatic cancer (see Figure 1). Because her 42-year-old cousin had already initiated genetic testing, DB was encouraged to contact the clinic again when her cousin's results had been obtained; if a mutation was detected in her cousin, then single-site testing for that same mutation could be performed for DB.
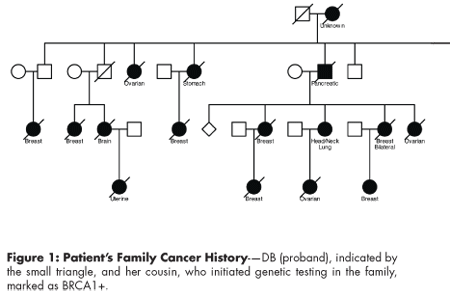
Click to enlarge
In March 2004, DB returned to the genetics department for genetic counseling and testing. The same advanced practice nurse who conducted her initial risk assessment and genetic counseling session reviewed the risks and benefits of genetic testing with DB, emphasizing the usefulness of having a known mutation in the family. DB consented to genetic testing and her blood was drawn. Results were obtained in late March 2004 and discussed with DB in a face-to-face disclosure session at the genetics clinic.
Options for reducing the risk of both breast cancer and ovarian cancer were reviewed. DB's greatest fear was ovarian cancer, and she was given a referral to see a gynecologic oncologist for evaluation of prophylactic oophorectomy. As discussed previously, DB did agree to proceed with the recommended procedure and had her surgery in July 2004. She was also advised about options for breast cancer risk reduction, including surveillance with MRI, mammogram, ultrasound and CBE; chemoprevention with tamoxifen; and prophylactic bilateral mastectomies with reconstruction. She chose surveillance procedures to monitor her breast tissue but opted to delay surveillance with MRI, owing to insurance reimbursement concerns.
DISCUSSION
Prior to April 2007, there were no standard recommendations for monitoring women at high risk for breast cancer. The American Cancer Society (ACS) suggested that "women at increased risk (eg, family history, genetic tendency, and past breast cancer) should talk with their doctors about the benefits and limitations of starting mammography earlier, having additional tests (eg, breast ultrasound or MRI), or having more frequent exams."[4] However, the ACS did not provide clear guidelines for defining high risk or for following high-risk women. This was the dilemma for DB, who received a recommendation she wanted to follow but was stymied by the realistic issue of the cost of care and insurance reimbursement.
In April 2007, the ACS published new guidelines that not only define high risk, but also state clear recommendations for following high-risk women. "Screening MRI is recommended for women with an approximately 20%–25% or greater lifetime risk of breast cancer, including women with a strong family history of breast or ovarian cancer and women who were treated for Hodgkin disease."[5]
The ACS changed their guidelines based on an analysis of studies performed in high-risk women that indicated the significant benefit of MRI for diagnosing breast cancer, and for diagnosing it at an earlier stage. Seeing women every 6 months was also found to be significant in reducing tumor size and, potentially, the stage of their breast cancer at diagnosis.
The risk of breast cancer is approximately 45%–65% in women with hereditary breast and ovarian cancer mutations,[6] which definitely puts these patients within ACS published guidelines for high risk. Identifying who these high-risk women are, counseling them, and offering genetic testing with appropriate follow-up, may very well save many lives.
OUTCOME
Since the ACS guidelines were published, we have reviewed the risk of each of our patients as they come in for high-risk appointments. Those who meet the criteria for MRI are highly encouraged to receive one. Our patients who are BRCA+ have a much better sense that their MRI will be covered by insurance, and coverage is generally no longer an issue for these patients. We have also incorporated MRI in the first screening for the newly diagnosed BRCA+ high-risk patient. We no longer wait until the next appointment (6 months later) to obtain a baseline MRI, and we have become much more direct in our recommendation to incorporate MRI in routine annual screening of hereditary breast and ovarian cancer patients. As to DB, she has completed her chemotherapy and is doing well.
References:
1. Ford D, Easton DF, Bishop DT, et al: Risks of cancer in BRCA1-mutation carriers. Breast Cancer Linkage Consortium. Lancet 343(8899):692–695, 1994.
2. Gail M, Brinton L, Byar D, et al: Projecting individualized probabilities of developing breast cancer for white females who are being examined annually. J Natl Cancer Inst 81(24):1879–1886, 1989.
3. Claus EB, Risch N, Thompson WD: Autosomal dominant inheritance of early-onset breast cancer: Implications for risk prediction. Cancer 73(3):643–651, 1994.
4. Smith RA, Saslow D, Sawyer KA, et al: American Cancer Society guidelines for breast cancer screening: Update 2003. CA Cancer J Clin 53(3):141–169, 2003.
5. Saslow D, Boetes C, Burke W, et al: American Cancer Society Guidelines for Breast Screening with MRI as an adjunct to mammography. CA Cancer J Clin 57(2):75–89, 2007.
6. Antoniou A, Pharoah PD, Narod S, et al: Average risks of breast and ovarian cancer associated with BRCA1 or BRCA2 mutations detected in case series unselected for family history: A combined analysis of 22 studies. Am J Hum Genet 72(5):1117–1130, 2003.