Myelodysplastic Syndromes: Diagnosis, Treatment Planning, and Clinical Management
Abstract Innovations in the diagnosis, risk stratification, and treatment of the myelodysplastic syndromes (MDS) have provided several new therapeutic options and renewed hope for patients with the disease. Optimal treatment requires careful evaluation of each patient using newly established criteria. Identifying the common symptoms in the MDS patient, integrating new therapies with novel mechanisms of anti-tumor activity and unique toxicity profiles, and developing tools to assist patients receiving these treatments have created unique challenges for the oncology nurse. Many of the emerging therapies have shown promise in tumor response and may be administered over extended periods of time. Most allow patients to be treated in an outpatient setting. This article will explore the diagnosis, treatment planning, and clinical management of patients with MDS.
Abstract Innovations in the diagnosis, risk stratification, and treatment of the myelodysplastic syndromes (MDS) have provided several new therapeutic options and renewed hope for patients with the disease. Optimal treatment requires careful evaluation of each patient using newly established criteria. Identifying the common symptoms in the MDS patient, integrating new therapies with novel mechanisms of anti-tumor activity and unique toxicity profiles, and developing tools to assist patients receiving these treatments have created unique challenges for the oncology nurse. Many of the emerging therapies have shown promise in tumor response and may be administered over extended periods of time. Most allow patients to be treated in an outpatient setting. This article will explore the diagnosis, treatment planning, and clinical management of patients with MDS.
The myelodysplastic syndromes (MDS) represent a spectrum of clonal stem cell malignancies that are characterized by dysplastic and ineffective hematopoiesis. They are associated with progressive cytopenias and a variable risk of bone marrow failure and leukemic transformation.[1,2] The exact cause of MDS is not clearly understood. MDS is thought to originate as a result of complex interactions between malignant progenitor cells (malignant clone), the bone marrow stroma, and the microenvironment.[3] In general, as the disease progresses, bone marrow function declines. Identification of key molecular, immunological, and hematological elements of the pathobiology of myelodysplastic syndromes has provided insight into potential therapeutic targets. Understanding the novel mechanisms of anti-tumor activity has promoted robust scientific discovery in terms of active therapies for this disease.
The first classification system for MDS was developed in 1976. A risk-stratification system, the International Prognostic Scoring System (IPSS), was developed in 1997, providing information critical for treatment selection.[4] The first agent for active treatment of MDS was approved by the US Food and Drug Administration (FDA) in May 2004. Since then, two additional active agents have been approved for MDS, and several other agents are in clinical trials.
The first clinical guidelines for treatment were released in July 2004 by the National Comprehensive Cancer Network. Several revisions have been made since their development, reflecting the rapidly changing treatment guidelines. Improvement in supportive care strategies, including hematopoietic growth factors, blood component support, iron-chelation therapies, and antibiotics, has in turn improved overall survival of patients with the disease. The disease is most common in elderly patients, thus presenting additional challenges for therapeutic management. The challenge for oncology nursing professionals is to integrate knowledge of selective therapies based on disease characteristics, risk stratification, and individual patient attributes.
CLINICAL PRESENTATION, DIAGNOSTIC EVALUATION, AND RISK STRATIFICATION
Symptoms associated with one or more cytopenias--such as fatigue, fever, recurrent or prolonged infections, bruising, or bleeding--are the most common symptoms that prompt the patient to seek medical care. The initial patient evaluation most often includes a complete blood count, which reveals normocytic or macrocytic anemia, normal to decreased neutrophils, and variable platelet counts. Anemia is observed in 90% of patients with MDS, either at initial presentation or during the course of their disease. A careful history and additional laboratory analysis should be pursued to exclude other causes of cytopenias. (See Table 1.)
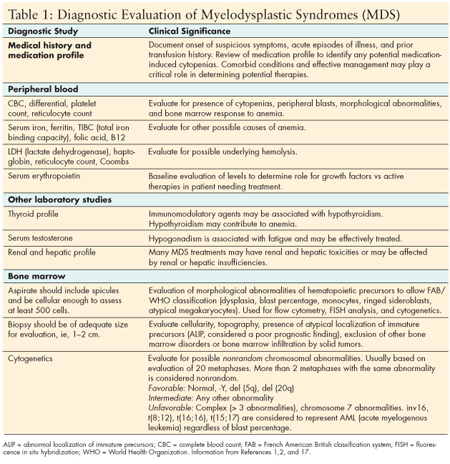
If MDS is suspected, a bone marrow biopsy and aspirate with cytogenetic analysis will be necessary to exclude other diseases associated with bone marrow failure and allow full classification and risk stratification of MDS. These procedures will yield a tissue diagnosis, information on morphology, cell counts, blasts percentage, bone marrow cellularity, any atypical findings, and cytogenetic evaluation data. This information is critical to identification of favorable or unfavorable subtypes and specific options for therapy.
Diagnostic classification
Diagnostic classification of MDS is based on the three primary classification systems: The French American British (FAB) classification system, the World Health Organization (WHO) system, and the International Prognostic Scoring System (IPSS). Collectively they provide guidelines for assignment of disease nomenclature and risk stratification based on hematopathology and cytogenetic criteria. (See Table 2.) Risk stratification is based on three criteria--percentage of blasts, number of cytopenias, and favorable or unfavorable cytogenetics.
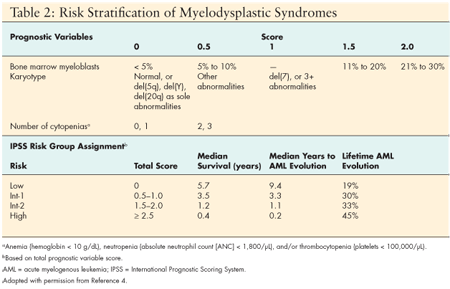
Low-risk disease (low – Intermediate 1) is associated with longer survival and less common transformation to acute leukemia. Treatment goals for the lower-risk patients are aimed at improving hematopoiesis and reducing the risk of cytopenias. High-risk disease (Intermediate 2 – High) is associated with rapid leukemic transformation and poor prognosis. Treatment is aimed at control of the aggressive component of the disease and improved survival. Consideration of diagnostic findings that may favor selection of specific therapies is critical to initiating the therapy with the greatest potential benefit early in the course of disease.
THERAPEUTIC GOALS AND TREATMENT SELECTION
There are challenges in trying to determine optimal treatment strategies for people with MDS. Until recently, many treatment strategies were based on anecdotes, abstracts and consultation meetings, and relatively few published clinical trials. Recent clinical trial results are encouraging but generalized treatment recommendations are limited by the different eligibility and response criteria used in the earlier trials. Continued refinement of response evaluation criteria such as the International Working Group (IWG) Criteria and consensus treatment guidelines such as NCCN criteria will provide a framework for comparison of clinical trial results in the future.
The primary goals of therapy are to improve quality of life, minimize treatment toxicity, decrease transfusions, decrease infections, and prolong survival. Selection of the most effective therapy should be based on the unique characteristics of the individual patient, including specific MDS subtype, risk category, performance status, physiological age, comorbid conditions, and lifestyle. Familiarity with recent advances in treatment options is essential to selecting the best therapy for the individual patient.
For low- to intermediate-risk MDS patients (those in the IPSS Low and Int-1 prognostic risk groups), blood counts may remain stable over several months or longer, therefore a short period of observation is recommended to determine a patient's degree of clinical stability. Monitoring every 2 to 4 months is recommended for patients in stable condition. Patients who develop progressive cytopenias or transfusion dependence are then considered for active treatment. Patients with high-risk disease require more aggressive and immediate intervention. (See Table 3.)
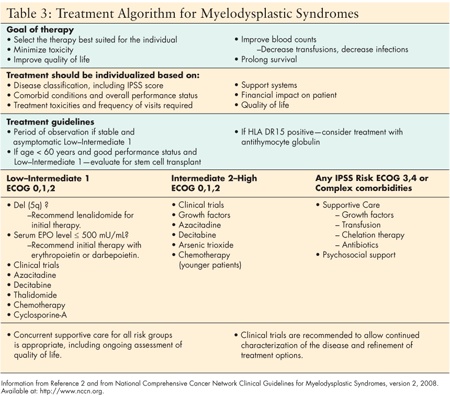
Supportive care has been the mainstay of MDS treatment for the last 20 years. Until recently, no active therapies were available. Supportive care continues to be important in treatment of symptoms associated with the disease or toxicities of newly developed active therapies. Supportive care includes observation, quality of life assessment, growth factors, antibiotics, iron-chelation therapies, and transfusion support. However, it is important to recognize that supportive care measures do not change the underlying disease and may also be associated with toxicity, cost, and frequent clinical evaluation or visits.
NURSING MANAGEMENT OF CYTOPENIAS
Cytopenias associated with MDS are a result of ineffective production of bone marrow cells. Progressive disease or bone marrow failure often prompts providers to initiate active therapies that cause further and often prolonged cytopenias prior to treatment response. This predisposes the patient to potential morbidity and mortality very similar to patients who have developed myelosuppression for other reasons due to peripheral destruction. The patient with MDS may have prolonged and more severe cytopenias resulting from both a production abnormality and peripheral destruction due to active therapy. The presence of chronic moderate to severe cytopenias requires careful nursing assessment for presenting signs and symptoms, and an understanding of the available treatment strategies and indications for their use. Most patients are effectively managed in an outpatient setting. (See Table 4.)
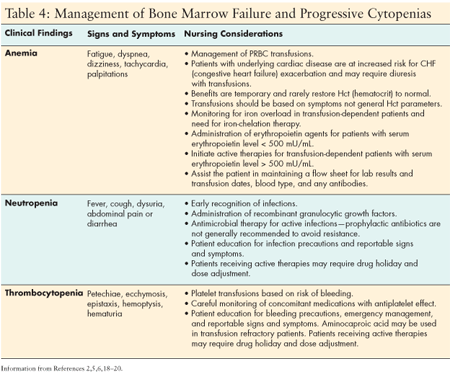
ANEMIA MANAGEMENT
Approximately 90% of patients with MDS become transfusion-dependent over the course of their disease. Transfusion dependence is most common in high-risk patients (79%) compared with low-risk patients (39%). PRBC (packed red blood cell) transfusions provide transient hemoglobin improvement and may provide the patient with temporary improvement in fatigue and other symptoms associated with anemia.[5] There are several potential risks associated with chronic transfusions, particularly in the elderly population with common underlying cardiovascular, pulmonary, or renal disease; these risk include volume overload and an increase in infectious complications. Over time, the patient may develop antibodies which make cross-matching potential donor units more difficult and cause delays in availability of blood products.
Recombinant erythropoietic growth factors may be beneficial in reducing transfusion dependence in a subset of MDS patients who have low endogenous levels (< 500 mU/mL) of erythropoietin (EPO) and require fewer than 2 units of PRBC transfusion per month. However, endogenous levels of EPO in patients with MDS are most often very high (> 500 mU/mL), limiting the number of patients who are likely to respond to treatment with these agents.[6]
Thromboembolic events remain a rare but seious adverse event with erythropoietin agents, warranting pretreatment risk assessment and continued surveillance.[7] Target hemoglobin levels should not exceed 12 g/dL. Levels higher than 12 g/dL have been associated with significant adverse events, including thromboembolic events, heart failure, and death in patients with chronic kidney disease not receiving dialysis; shortened time to tumor progression in head and neck cancer patients receiving radiation therapy; and shortened survival in metastatic breast cancer patients receiving chemotherapy.[8]
Though no similar data have been reported in patients with MDS, based on these findings new recommendations for their use have been made by the FDA.[9] Guidelines for use of these agents in nonrenal disease indications have been established by the Centers for Medicaid Services (CMS) and may vary regionally for Medicare Fiduciaries (CMS Decision Memo, available at: https//www.cms.hhs.gov/mcd/viewdecisionmemo.asp?id=203).
THROMBOCYTOPENIA MANAGEMENT
Thrombocytopenia presents a challenge similar to that of anemia. Although transfusion dependence is less common, many patients develop moderate sustained thrombocytopenia which is asymptomatic. In these patients, a focus on bleeding precautions, safety precautions to reduce potential trauma, and reducing potential risks associated with medications that inhibit platelet function are the most important interventions. Though guidelines for transfusion of platelets vary by institution, a platelet count below 10,000 per microliter of blood significantly increases the risk of a spontaneous bleed and is often the recognized indication for transfusion. Patients with comorbid conditions may need higher parameters for transfusions. Aminocaproic acid may be considered in patients who become refractory to platelet transfusions and exhibit bleeding tendencies.
NEUTROPENIA MANAGEMENT
Neutropenia in MDS (absolute neutrophil [ANC] count < 1,500 mm3) is common and may be chronic. Patients are at an increased risk of infections, primarily those common in older patients. Prophylactic use of recombinant growth factors is generally not recommended owing to the temporary improvement seen, the associated expense, and the frequency of office visits required. Prophylactic antibiotics are also not recommended, owing to concern about development of resistance and the potential for drug reactions.
In the presence of an active infection or in response to treatment-induced neutropenia, growth factors are recommended and patients generally respond readily with improvement in the ANC. Certain active therapies used in the treatment of MDS affect rapidly dividing cells and same-day administration of white blood cell (WBC) growth factors is contraindicated. Bone pain associated with the agents is less common in the MDS patient, as it is uncommon to see WBC responses above 10,000 / mm3.
IRON OVERLOAD
Continued RBC transfusion dependence leads to iron accumulation in the heart, the liver, and the adrenal glands. Symptoms are often vague and may not be evident until the patient has developed serious organ damage. Elevated hepatic enzymes, mild symptoms of congestive heart failure, development of diabetes, or vague abdominal bloating and pain may indicate the need to evaluate the patient with MRI (magnetic resonance imaging) of the liver or diagnostic echocardiogram. Patients with MDS should have a baseline serum ferritin level checked and have this test repeated periodically. A recent study by Armand et al of 590 patients undergoing allogeneic transplantation found an elevated serum ferritin level prior to transplant was associated with lower overall survival, and in patients with MDS was attributed to treatment-related mortality.[10] Veno-occlusive disease was more common in these patients. A study conducted by Malcovati et al evaluated the prognostic factors and life expectancy in 467 MDS patients with clinically relevant iron burden (ferritin >1000 mg/mL).[11] Transfusion-dependent patients had significantly shorter survival times (P < .001). The primary factor associated with shorter survival time was the number of units received per month. Cardiac failure occurred in 51% of the patients and infections occurred in 31% of patients. Hemorrhage (8% of patients) and hepatic cirrhosis (8%) were also reported.
Two FDA-approved agents for iron chelation are available: deferoxamine (Desferal) and deferasirox (Exjade). Both agents bind iron in circulation and in the tissue, and the iron-bound form can be excreted efficiently in the urine and bile. A serum ferritin level > 2,500 mg/L is recognized by the NCCN as an indication to institute treatment. Deferoxamine is given intravenously or subcutaneously, most often via an infusion pump over 8–12 hours because of its very short half-life. Deferasirox is an effervescent tablet that is dissolved in water and taken by mouth on an empty stomach, 30 minutes before a meal each day. Recent data suggest mild neutropenia may be associated with administration of these agents. Additional toxicities include visual and auditory abnormalities, transient hepatic enzyme elevation, and, less commonly, elevated creatinine levels.
ACTIVE THERAPIES
Three active agents have been approved by the FDA since May 2004. They are 5-Azacitadine (Vidaza), lenalidomide (Revlimid), and decitabine (Dacogen). Clinical trial results indicate evidence of cytogenetic responses, suggesting that these agents do affect the underlying disease. Selection of the most appropriate agent is based on a number of factors, including the IPSS score, performance status, cytogenetics, and patient-specific characteristics. (See Table 3.) Ongoing clinical trials of these agents and other therapeutics offer continued hope for improved outcomes in treatment of MDS.
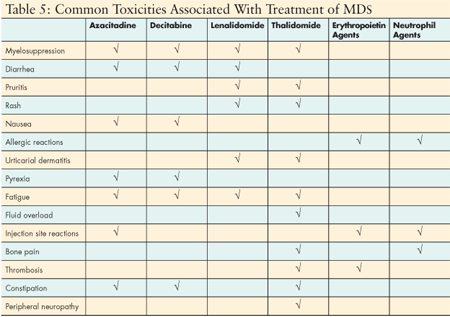
Each of these agents is associated with some toxicity. Myelosuppression is the most common side effect for all active therapies in MDS. In addition, most active therapies require sustained treatment over 2–4 months to induce a measurable response such as transfusion independence. Support of the patient with cytopenias induced by treatment is critical to sustaining therapy long enough to realize the benefit. Drug-specific administration requirements, toxicities, and monitoring requirements must also be considered in treatment selection and nursing management. (See Table 5.)
SPECIAL CONSIDERATIONS FOR THE ELDERLY POPULATION
Managing MDS is complicated by the generally advanced age of the patients (median age ranges from 65 to 70 years), presence of nonhematologic comorbid conditions, and the potential inability of the older patient to tolerate certain intensive forms of therapy. Elderly patients commonly have multiple medical problems, use medications to manage them, and are more likely to have more than one health care provider involved in their care; these factors all increase the risk of drug interactions and treatment toxicities.[12,13] Manifestations of common toxicities or illnesses may be more subtle in the elderly, owing to age-associated functional deficits in multiple organ systems.[14] Particularly important to the elderly MDS patient is the age-related decline in normal bone marrow function, including diminished capacity for response to stressors such as infection or myelosuppressive treatments.[15,16]
Integrating geriatric and oncology nursing strategies allows an individualized approach toward this unique population. As with many diseases in the elderly, reliance on family members or friends to maintain the prescribed treatments, including travel to appointments, may place additional stressors on the patient and his or her support network. Careful evaluation of functional status, ability to tolerate treatments, effect of disease progression, and general overall health can provide the best opportunity for nursing support of these patients. Assessment of activities of daily living may detect deficiencies or deficits that require early intervention before they become problematic.
SUMMARY AND FUTURE TRENDS
The myelodysplastic syndromes affect primarily an older population. The only curative therapy, allogeneic bone marrow transplant, is not an option for most patients. MDS is one of many diagnoses with a rapidly changing treatment paradigm based on scientific advances and clinical management strategies. Several new agents recently approved or in clinical trials show promise in terms of affecting the natural progression of this disease. Supportive care should continue concurrently with active therapies to reduce symptoms. Familiarity with the disease pathobiology, optimal diagnostic evaluation, and emerging treatment strategies will assist the oncology nurse in clinical management of the MDS patient.
References:
1. List AF, Sandberg AA, Doll DC: Myelodysplastic Syndromes, in Lee GL, Bithell T, Forester J, et al (eds). Wintrobes Clinical Hematology. 11th ed. Philadelphia, Pennsylvania, Lippincott Williams and Wilkins, 2004, pp 2207–2234.
2. Kurtin SE: Advances in the management of low to intermediate risk myelodysplastic syndrome: Integrating the National Comprehensive Cancer Network Guidelines. Clin J Oncol Nurs 10(2):197–208, 2006.
3. List AF: New approaches to the treatment of myelodysplasia. Oncologist 7(suppl 1):39–49, 2002.
4. Greenberg C, Cox MM, LeBeau P, et al: International scoring system for evaluating prognosis in myelodysplastic syndromes. Blood 89:2079–2088, 1997.
5. Casadevall N, Durieux P, Dubois S, et al: Health, economic and quality of life effects of erythropoietin and granulocyte colony stimulating factor for the treatment of myelodysplastic syndromes: A randomized controlled trial. Blood 104:321–327, 2004.
6. Hellström-Lindberg E: Approach to anemia associated with myelodysplastic syndromes. Curr Hematol Rep 2:122–129, 2003.
7. Kurtin S: A time for hope: Promising advances in the management of anemia, neutropenia, thrombocytopenia, and mucositis. J Support Oncol 5(suppl 2):085–088, 2007.
8. Amgen's letter to healthcare professionals. Available at: http://www.amgen.com/pdfs/misc/healthcare_professionals_letter.pdf. Accessed March 19, 2007.
9. US Food and Drug Administration. FDA Health Advisory-Erythropoiesis-stimulating agents (ESAs): Epoetin alfa (marketed as Procrit, Epogen) and darbepoetin alfa (marketed as Aranesp). Available at: http://www.fda.gov/cder/drug/advisory/RHE2007.htm. Accessed March 19, 2007.
10. Armand P, Kim H, Cutler C, et al: Prognostic impact of elevated pre-transplant serum ferritin in patients undergoing myeloablative stem cell transplantation. Blood 190(10):4586–4588, 2007.
11. Malcovati L, Porta MG, Pascutto C, et al: Prognostic factors and life expectancy in myelodysplastic syndromes classified according to WHO criteria: A basis for clinical decision making. J Clin Oncol 23(30):7594–7603, 2005.
12. Balducci L: The elderly patient with cancer: New approaches for improved outcomes. J Support Oncol 1:4(suppl 2):3–4, 2003.
13. Boyle D: Cancer in the elderly: Key facts. Oncol Support Care Q 2(1):6–21, 2003.
14. Green JM, Hacker ED: Chemotherapy in the geriatric population. Clin J Oncol Nurs 8:591–597, 2004.
15. Berger A: Bone marrow function decline with aging. Oncol Support Care Q 2(1):22–31, 2003.
16. Van Cleave J: Supportive care of the elderly patient with cancer. Oncol Support Care Q 2(1):44–59, 2003.
17. Vardiman JW: Hematopathological concepts and controversies in the diagnosis and classification of myelodysplastic syndromes. Hematology Am Soc Hematol Educ Program 2006:199–204.
18. Gupta P, Leroy S, Luikart S, et al: Long-term blood product transfusion support for patients with myelodysplastic syndromes (MDS): Cost analysis and complications. Leuk Res 23:953–959, 1999.
19. Hershko C, Graham G, Bates G, et al: Non-specific serum iron in thallassemia: An abnormal serum iron fraction of potential toxicity. Br J Haematol 40:255–263, 1978.
20. Ludwig H: Anemia of hematological malignancies: What are the treatment options? Semin Oncol 29:45–54, 2002.