Hypothyroidism Enhances High-Dose Tamoxifen in Glioma
SAN FRANCISCO-Although tamoxifen (Nolvadex) kills glioma cells in culture, it has not been effective in prolonging survival in patients with recurrent glioma. A study presented at the 93rd Annual Meeting of the American Association for Cancer Research (abstract 2442), however, found that pretreating with propylthiouracil and Lugol’s solution (a solution of potassium iodide and iodine) to induce chemical hypothyroidism prior to high-dose tamoxifen therapy resulted in dramatic increases in survival time in these patients.
SAN FRANCISCOAlthough tamoxifen (Nolvadex) kills glioma cells in culture, it has not been effective in prolonging survival in patients with recurrent glioma. A study presented at the 93rd Annual Meeting of the American Association for Cancer Research (abstract 2442), however, found that pretreating with propylthiouracil and Lugol’s solution (a solution of potassium iodide and iodine) to induce chemical hypothyroidism prior to high-dose tamoxifen therapy resulted in dramatic increases in survival time in these patients.
"In research that we did at the Cleveland Clinic, we found that in cell cultures insulin growth factor-1 (IGF-1) counteracts the cell-killing effect of tamoxifen," said Aleck Hercbergs, MD, a radiation oncologist at the Cleveland Clinic. Since triiodothyroxine regulates IGF-1, he continued, "we reasoned that suppressing thyroid gland activity to a low, but clinically tolerable, level would reduce IGF-1 levels and thus increase the effectiveness of tamoxifen."
In the clinical study, 38 patients with recurrent glioma were treated with up to 1,000 mg of propylthiouracil daily and 30 mg of Lugol’s solution three times daily for 14 days to induce hypothyroidism, defined as a free thyroxine level of less than 0.7 ng/dL. Only half of the patients developed hypothyroidism after a median of 26 days. The other 19 remained euthyroid. Dr. Hercbergs hypothesized that all of the 38 patients would have eventually developed hypothyroidism if they had lived long enough. The tamoxifen dosage was 240 mg/d.
Median survival time for the hypothyroid patients was 10.6 months, and two of these patients were still alive after 3 years. Median survival time for the euthyroid patients was 3.7 months, with only one patient in this group living longer than 8 months.
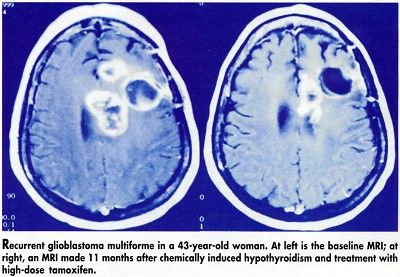
Based on MRIs, five of the hypothyroid patients experienced a greater than 50% reduction in tumor size, compared with none of the euthyroid patients (see Figure). Interestingly, one patient who became hypothyroid showed a partial response even though he could not continue to take tamoxifen due to ataxia.
IGF-1 levels were significantly reduced in the hypothyroid group, compared with the euthyroid group (P < .005). However, IGF-1 levels were measured in only seven hypothyroid patients and three euthyroid patients.
Most of the reported side effects, such as deep vein thrombosis, were typical of those seen in glioma patients. "There was one patient who experienced possible cold intolerance, but this was in Cleveland in the winter," Dr. Hercbergs said, so it was unclear if this was due to hypothyroidism.
"Chemically induced hypothyroidism significantly improves survival and response to tamoxifen in recurrent high-grade glioma in association with reduced blood IGF-1 levels," Dr. Hercbergs concluded. "We suggest that the thyroid may be an important intrinsic modulator of malignant glioma progression and that this approach may be useful as complementary therapy in other sorts of malignancies."
Validation Needed
At an AACR news briefing, Daniel Karp, MD, pointed to anecdotal cases in the literature over the last 20 or 30 years of breast cancer patients who became hypothyroid and subsequently did much better than expected. Dr. Karp, deputy head of cancer medicine, M.D. Anderson Cancer Center, called the research "very exciting and biologically oriented," but added that it needs to be validated.
In an interview, Dr. Hercbergs agreed that further studies are needed to elucidate the role of thyroid hormone in tumor growth and the usefulness of hypothyroidism in prolonging survival. He suggested that, in the meantime, oncologists could use their clinical judgment in deciding if they should correct thyroid deficiencies in cancer patients who become hypothyroid while under their care.
"Treat the patient, not the blood test," Dr. Hercbergs said. In the absence of validating studies, clinicians could at least delay thyroid treatment in those hypothyroid patients who are not experiencing clinical effects. Researchers at the Cleveland Clinic are currently studying the effectiveness of this combination therapy in newly diagnosed glioma patients.