Imaging Tumoral Hypoxia: Oxygen Concentrations and Beyond
The role of hypoxia as a key determinant of outcome for human cancers has encouraged efforts to noninvasively detect and localize regions of poor oxygenation in tumors. In this review, we will summarize existing and developing techniques for imaging tumoral hypoxia. A brief review of the biology of tumor oxygenation and its effect on tumor cells will be provided initially. We will then describe existing methods for measurement of tissue oxygenation status. An overview of emerging molecular imaging techniques based on radiolabeled hypoxic markers such as misonidazole or hypoxia-related genes and proteins will then be given, and the usefulness of these approaches toward targeting hypoxia directly will be assessed. Finally, we will evaluate the clinical potential of oxygen- and molecular-specific techniques for imaging hypoxia, and discuss how these methods will individually and collectively advance oncology.
The role of hypoxia as a key determinant of outcome for human cancers has encouraged efforts to noninvasively detect and localize regions of poor oxygenation in tumors. In this review, we will summarize existing and developing techniques for imaging tumoral hypoxia. A brief review of the biology of tumor oxygenation and its effect on tumor cells will be provided initially. We will then describe existing methods for measurement of tissue oxygenation status. An overview of emerging molecular imaging techniques based on radiolabeled hypoxic markers such as misonidazole or hypoxia-related genes and proteins will then be given, and the usefulness of these approaches toward targeting hypoxia directly will be assessed. Finally, we will evaluate the clinical potential of oxygen- and molecular-specific techniques for imaging hypoxia, and discuss how these methods will individually and collectively advance oncology.
The presence of hypoxic cells within tumors and the implications of this situation for cancer therapy were first noted over 50 years ago by Thomlinson and Gray.[1] Since that time, a plethora of studies have confirmed the existence of hypoxic cells within a variety of tumor types as well as the prognostic importance of hypoxia in the clinical management of human cancers. The role of hypoxia as a key determinant of outcome for human cancers has encouraged efforts to noninvasively detect and localize regions of poor oxygenation in tumors. In this review, we will summarize existing and developing techniques for imaging tumoral hypoxia.
Biology of Tumor Hypoxia
Most tumors possess (1) lower oxygen levels than their corresponding tissue of origin, (2) significant intra- and intertumoral variation in oxygen concentrations, and (3) lower oxygenation at the time of recurrence than the corresponding primary tumor.[2,3] Hypoxic tumors are more refractory to therapy and are associated with an aggressive tumor phenotype. For example, the "oxygen effect" has been well studied and dictates that the radiation dose required to kill a given fraction of cells in a cell population fully deprived of oxygen is 2.5 to 3 times greater than that required to achieve an equal amount of cell kill in an aerobic cell population.[4-6] Hypoxia-induced resistance to chemotherapy including cyclophosphamide, carboplatin, and doxorubicin,[7,8] have also been noted. Tumor hypoxia has been associated in both laboratory and clinical studies with a more aggressive neoplastic phenotype as well as with increased potential for invasion, growth, and metastasis.[9-16]
A cascade of alterations in cellular gene expression are induced by the absence of oxygen. A central player in the ability of tumor cells to adapt to a low oxygen environment is the hypoxia-inducible factor 1 (HIF-1). HIF-1 is a heterodimeric protein consisting of an oxygen-regulated alpha subunit and a constitutively expressed beta subunit.[17] In the presence of oxygen, a family of prolyl hydroxylases are activated and hydroxylate two proline residues of HIF-1-alpha (P402 and P564).[18-20] Following hydroxylation, HIF-1-alpha is then further modified by the addition of ubiquitin molecules through association with a complex consisting of the von-Hippel Lindau (VHL) protein and elongins B and C,[21] after which the protein is rapidly degraded by the proteasome. In cells with wild-type expression of all components of this pathway, HIF-1-alpha therefore only persists under hypoxic conditions where the oxygen-dependent degradation pathway is inactive due to inhibition of the prolyl hydroxylases that require molecular oxygen. In these situations, HIF-1-alpha combines with HIF-1-beta to generate the composite transcription factor HIF-1, and selectively activate transcription of genes bearing promoters containing HIF-1-binding hypoxia-response elements (HREs).
A number of clinical studies have shown that increased expression of HIF-1-alpha is a significant negative prognostic indicator for many types of cancer, including brain,[22,23] breast,[24-26] cervix,[27-29] esophagus,[30] head and neck,[31,32] and uterus.[33] These observations have encouraged the development of a variety of anti-HIF-1 cancer therapeutics.[34,35] It has furthermore been observed that HIF-1-alpha can be found in oxic as well as hypoxic conditions in many types of tumor cells.[36] This finding provides evidence of oxygen-independent regulation of HIF-1 in tumor cells, a process that is partly mediated by loss and/or gain of function mutations in a number of genes relating to the degradation pathway described above, such as VHL.[34,35]
A variety of growth factors including insulin and insulin-like growth factor 1 (IGF-1) have also been shown to stimulate oxygen-independent accumulation of HIF-1 through the phosphatidylinositol 3-kinase (PI3K)/Akt/mTOR pathway.[37-39] Overexpression of HIF-1 and its downstream genes, including vascular endothelial growth factor (VEGF), glucose transporter 1 (GLUT-1), lysyl oxidase (LOX), and carbonic anhydrase IX (CA IX), appears to be at least partly responsible for the phenotype of increased aggressiveness, invasion, metastasis, and resistance to therapy associated with hypoxic tumor cells.[35,40]
Gatenby and Gillies have speculated that hypoxic conditions in premalignant lesions may select for tumor cells overexpressing HIF-1 because of the survival advantage it provides.[41] This could produce adult tumors with elevated HIF-1 levels under both oxic and hypoxic conditions that would therefore constitutively exhibit an aggressive phenotype. Two recent experimental studies have suggested that the absence of HIF-1-alpha confers therapeutic sensitivity independent of tissue oxygen levels, indicating that HIF-1-alpha is an effector and not merely a surrogate of hypoxia-mediated therapeutic resistance.[42,43] Thus, HIF-1 and hypoxia represent two related but independently significant aspects of cancer biology. Correspondingly, imaging methods and therapies targeting HIF-1 and/or HIF-1-alpha in tumors, while not necessarily specific to oxygenation status, may nonetheless be directed toward neoplastic cells with a more malignant phenotype.
Measuring Tumoral Oxygenation
Invasive Approaches
A variety of techniques have been advanced for the identification and quantification of tumoral hypoxia. A compilation of these methods is given in Table 1. The past gold standard for measurement of oxygen levels in tumors is the Eppendorff needle electrode, which applies a voltage to cause the reduction of molecular oxygen to hydroxide. The current produced is then measured and related to the concentration of oxygen within the measurement volume.[44] Alternative probe-based oxygen measurement strategies have been developed, including systems based on oxygen-sensitive fluorophores such as the OxyLite.[45]
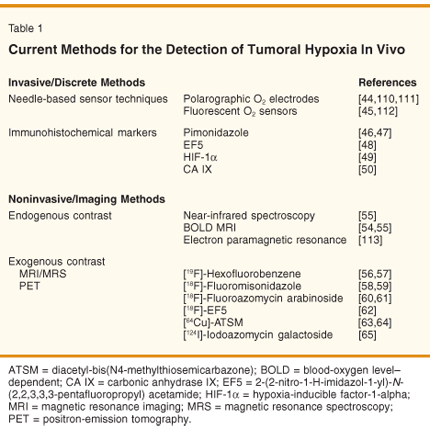
In addition, immunohistochemical methods have been applied to detect markers of hypoxia in biopsy samples taken from cancer patients. These markers include both exogenous, systemically delivered probes that are administered to a patient, localize in hypoxic regions, and are detected by antibody techniques in tissue specimens (pimonidazole,[46,47] EF5[48]), as well as endogenous proteins that are overexpressed under hypoxic conditions (HIF-1-alpha,[49] CA IX[50]). These methods bear restrictions to their applicability and utility because of their invasive nature, the possibility of sampling error in relating discrete and/or microscopic observations to a macroscopic, potentially heterogenous tumor, and the inability to perform serial measurements needed to assess changes in oxygenation during treatment.
Noninvasive Methods
Imaging-based techniques for the measurement of tissue hypoxia in vivo present the opportunity to construct a spatial map of oxygen concentration, thereby obtaining a more complete picture of the physiologic environment of a tumor. The noninvasive nature of these methods makes them amenable to serial administration, facilitating observation of changes in oxygenation over time as an individual cancer grows and receives treatment. Furthermore, images of tissue oxygen partial pressure could in principle be used to target irradiation and/or other focal therapies to the most hypoxic regions of the tumor, which are expected to be the most resistant to treatment.
Noninvasive methods for detecting hypoxia either exploit intrinsic oxygen-dependent contrast mechanisms, or rely upon administered imaging agents that selectively accumulate or become activated within hypoxic cells and/or tissues. Of the former, several techniques have been developed that utilize the distinct biochemical properties of oxy- and deoxyhemoglobin. Alterations in the near-infrared absorption spectrum of hemoglobin before and after binding oxygen have suggested the use of optical spectroscopy[51,52] and tomography[53] to quantify blood oxygen saturation. Oxy- and deoxyhemoglobin also exhibit differences in their relaxation properties following exposure to a magnetic field. This phenomenon forms the basis of blood oxygen level-dependent (BOLD) contrast in functional magnetic resonance imaging (fMRI), and these techniques have therefore been applied toward measuring blood oxygen saturation in tumors.[54,55]
Probe-based strategies for detecting hypoxia can employ either positive or negative contrast, corresponding to whether imaging signals correlate with oxygen concentration or with hypoxia. Because the primary aim of these efforts is to detect hypoxic regions in tumors and because of the inherent limitations of negative contrast imaging, most methods have sought to generate increased imaging signals in regions of low oxygenation. Magnetic resonance imaging (MRI) approaches using contrast agents have employed compounds whose relaxivity is dependent on the concentration of dissolved oxygen. In particular, [19F]-hexafluorobenzene has emerged as an agent that allows absolute determination of tissue oxygenation in vivo through fluorine-19 MRI and magnetic resonance spectroscopy (MRS).[56,57]
Nuclear medicine techniques for detecting tumor hypoxia have been in development for over 20 years, and have benefitted from the continued emergence of these systems in clinical medicine. Several types of molecules have demonstrated specific accumulation in hypoxic cells, notably the 2-nitroimidazoles which have formed the basis of many of these development efforts. A number of compounds of this type, including pimonidazole and EF5, have been used as immunohistochemical markers of hypoxia after visualization with specific antibodies. Positron- and gamma-emitting radioisotopes have been conjugated to these molecules, producing hypoxia-specific radiotracers for nuclear medicine. Agents of this class include [18F]-Fluoromisonidazole (FMISO),[58,59] [18F]-fluoroazomycin arabinoside (FAZA),[60,61] [18F]-2-(2-nitro-1-H-imidazol-1-yl)-N-(2,2,3,3,3-pentafluoropropyl) acetamide (EF5),[62] [64Cu]-diacetyl-bis(N4-methylthiosemicarbazone) (Cu-ATSM),[63,64] and [124I]-iodoazomycin galactoside.[65] These compounds reflect the ongoing quest for an agent with optimal biodistribution and sensitivity and specificity to hypoxic cells, while the different radioisotope labels allow tailoring of the probe imaging lifetime, in terms of radionuclide half-life, to the pharmacokinetic characteristics of the compound.
Imaging Downstream Effects of Hypoxia
As described above, the physiologic effects of hypoxia in terms of regulating gene expression and inducing changes in cellular behavior may provide a useful and potentially complementary target for cancer imaging and therapy. The advent of in vivo molecular imaging strategies for visualizing specific physiologic signatures in living subjects has allowed the development of several techniques for accomplishing detection of hypoxia-associated gene expression in vivo. Below we will summarize these efforts, identifying those primarily limited to laboratory studies and those with the potential for clinical translation.
Experimental Approaches
The concept of transfecting cells with exogenous "reporter genes" to allow measurement of cell location, metabolism, and/or activity in histologic samples is a well established technique, with beta-galactosidase[66] and the family of fluorescent proteins derived from Aequorea victoria,[67] including green fluorescent protein (GFP), being popular marker genes. With the emergence of macroscopic optical imaging technologies, GFP-expressing cells can now be imaged in vivo.[68] Similar imaging systems can also be used to perform whole-body imaging of cells expressing one of a family of enzymes known as luciferases, which act on corresponding members of a class of substrates known as luciferins.[69-71] In the oxygen-dependent luciferase reaction, a photon is generated that may be subsequently detected and used to localize and quantify the expression of luciferase in the subject.
DNA constructs encoding GFP and luciferase are optical marker genes that produce proteins that are directly responsible for the generation of an imaging signal. To detect gene expression by other imaging modalities, it is typically necessary to use the reporter gene as an effector of the accumulation or activation of an independent agent that generates imaging signals. Reporter gene systems for nuclear medicine have been devised that use a specific gene product to cause intra- or pericellular accumulation of an injected radiopharmaceutical. Herpes simplex virus type 1 thymidine kinase 1 (HSV1-tk) has been used as a vehicle to detect gene expression through positron-emission tomography (PET). Radiolabeled thymidine analogs labeled such as 9-(4-[18F]-3-hydroxy-methyl-butyl)guanine (FHBG) and 2´-[18F]-2´-deoxy-1-beta-d-arabionofuranosyl-5-iodo-uracil (FIAU) are injected intravenously into a subject. These compounds are then phosphorylated within cells expressing HSV1-tk, causing the tracer to selectively accumulate in these reporter-positive cells.[72-74] This accumulation can then be detected using PET imaging.
Similar approaches have been applied towards observing HIF-1-induced gene expression in vivo through the use of reporter genes that contain hypoxia responsive elements. Serganova et al constructed a reporter plasmid containing a fusion thymidine kinase/GFP reporter driven by a promoter containing eight tandem repeats of the HRE sequence, together with a constitutive CMV-driven RFP marker.[75] This construct was used to observe HIF-1 activation through microscopic fluorescence imaging and macroscopic PET imaging using FIAU. Imaging data were acquired over the course of tumor growth as well as after ischemically induced acute hypoxic challenges in three-dimensional cell spheroids and mouse C6 tumor models.
Expression of this reporter has also been compared with uptake of the PET hypoxia radiotracer FMISO in rat R3327-AT prostate carcinoma tumors grown in nude mice.[76] Hiraoka and colleagues have constructed HRE-driven fluorescent[77] and bioluminescent reporter constructs[78] to monitor hypoxia in murine tumors and the effectiveness of treating this tumor subpopulation using a hypoxia-directed prodrug chemotherapy strategy. Other investigators have exploited the oxygen-dependent degradation mechanism of HIF-1 to create fusion reporter proteins bearing the proline-containing oxygen degradation domain (ODD). Safran et al employed this approach to create a bioluminescent reporter that is regulated by oxygen at the posttranslational level.[79] A transgenic mouse expressing this reporter was then used to probe tissue-specific HIF-stabilization patterns and the effects of erythropoietin inhibitors.
A key issue with these approaches is the sensitivity of the reporter proteins to cellular oxygen concentrations. Many reporter proteins require oxygen as a cofactor for imaging signal generation, notably luciferase and green fluorescent protein. A recent comparison of the oxygen sensitivities of popular in vivo reporter genes verified that luciferase suffers from excessive signal loss in the absence of oxygen.[80] The findings cited above for HRE-driven luciferase reporters therefore suggest that overexpression of this reporter under hypoxic conditions outweighs hypoxia-mediated signal loss. However, this complicates the use of such a technique as a quantitative metric for HIF-1 activity. Continued development of oxygen-insensitive reporter strategies as well as methods for differentiating measurement of reporter expression from possible confounding effects of oxygenation will be needed to more rigorously assess the role of HIF-1 in cancer in vivo.
Clinically Relevant Approaches
Clinical studies of HIF-1 have relied upon detection and quantitation of the amount of this protein in tumors through immunohistochemical analysis of tumor biopsies using antibodies against HIF-1-alpha. Such methods are limited in their utility because (1) microscopic biopsy specimens do not offer a complete picture of the macroscopic tumor, and (2) one commonly cannot obtain serial biopsies over the course of treatment. Reporter gene techniques such as those described above have limited capacity for translation because of the difficulties in efficiently and stably transfecting an intact human tumor with a reporter gene construct. A more clinically relevant method of imaging HIF-1 would be to target it with a systemically administered imaging probe.
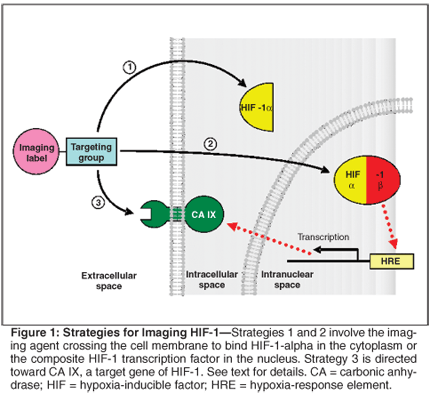
Figure 1 describes three potential strategies for achieving HIF-1-specific localization of such a probe. The first two approaches involve the imaging agent crossing the cell membrane to bind either HIF-1-alpha in the cytoplasm, or the composite HIF-1 transcription factor in the nucleus. Development of agents that perform these tasks is difficult because of the complexities of facilitating both passage of the agent across membranes as well as rapid clearance from cells without HIF-1-alpha and/or HIF-1. Strategy 3 examines an alternate method of detection of HIF-1 activity. Instead of targeting the imaging probe directly to HIF-1-alpha or HIF-1, it is instead directed toward CA IX, a cell membrane enzyme that is transcriptionally regulated by HIF-1 through an HRE in its promoter. As the probe can target and bind the extracellular portion of the protein, it is not hampered by the difficulty of crossing the cell membrane. In this respect, CA IX can be thought of as an endogenous reporter gene for HIF-1, one that is suitable for imaging using clinically relevant methods.
The carbonic anhydrases (CAs) are a family of enzymes that catalyze the reversible hydration of carbon dioxide to generate carbonic acid. CA IX, along with the other members of the CA enzyme family, is involved in a variety of physiologic tasks including respiration, pH and CO2 homeostasis, electrolyte secretion, and biosynthesis.[81] An analysis of CA IX expression in mice has demonstrated the presence of mRNAs for this protein in the stomach, small intestine, and colon, as well as in skeletal muscle and kidney cells. However, the protein is expressed in moderate amounts only in the stomach and liver, suggesting tissue-specific posttranscriptional regulation.[82] In humans, CA IX is expressed at low levels in normal tissues, with northern blot analysis of tissue extracts revealing moderate levels of CA IX mRNA in only the heart, stomach, liver, pancreas, and salivary gland.[50]
Over the past 15 years, there has been increasing interest in CA IX because of its association with a variety of types of cancer. Expression of this cell-surface CA isoform has been correlated with prognosis in a variety of tumor types.[83-89] Overexpression of this enzyme in tumors is thought to facilitate the characteristic low pH of the tumor extracellular space, which in turn expedites tumor growth and invasion by activating extracellular matrix metalloproteinases. Experiments in CA IX-deficient cell lines transfected with the gene and with CA IX-competent cells transfected with a small interfering RNA to silence expression of this protein have demonstrated the functional importance of CA IX in tumor survival and growth.[90]
Several antibodies to CA IX have been generated, including G250 and M75.[91,92] Further engineering of these antibodies has produced a humanized chimeric form of G250[93] as well as a number of variants of M75 specific to different epitopes of wild-type and mutant CA IX.[94] The chimeric cG250 antibody against CA IX has been radiolabeled and evaluated as an agent for imaging as well as radioimmunotherapy for renal cell cancers with high expression of CA IX. Preclinical investigations of radiolabeled cG250 in rats bearing renal cell xenograft tumors have demonstrated that this agent achieves tumor-to-blood ratios of ~3 at 72 hours postinjection.[95] A number of clinical trials of cG250 labeled with iodine-131 have been conducted and exhibited a positive therapeutic effect in the treatment of renal cancers.[96-98] These findings suggest localization of the radiolabeled antibody within the tumor and delivery of therapeutic doses of radiation while sparing other organs, conclusions that have been supported by radioimmunoscintigraphy imaging of the radiolabeled antibodies.
Engineered fragments of this antibody have also recently been developed and investigated as methods for targeting imaging and therapeutic agents, with similar pharmacokinetic profiles.[99] Chrastina and colleagues have studied M75 antibodies labeled with iodine-125 as a potential diagnostic and therapeutic agent.[100] The binding affinity of this antibody, which recognizes an epitope in the extracellular proteoglycan domain of CA IX, was measured to be 1.5 nM, with approximately 2.4 X 105 molecules of 125I-M75 binding to a single hypoxic HT-29 colon adenocarcinoma cell in vitro. While antibodies facilitate the targeting of CA IX in tumors with high specificity, this method of localizing drugs and/or imaging probes requires circulation times on the order of 2 to 4 days in order to achieve optimal target-to-background ratios. This limits the type of labels that may be conjugated to the antibodies to generate CA IX imaging probes to long-lived radioactive isotopes (copper-64, iodine-124) or nondecaying signal-generating groups such as fluorophores.
Small-molecule inhibitors of CA IX have also been developed that may prove useful as starting compounds for the development of imaging probes against this protein. Compounds containing a terminal sulfonamide group are potent inhibitors of the carbonic anhydrases because the sulfonamide group binds to to the zinc ion and hydrogen bonds with several amino acid residues within the enzyme's active site.[101] Some molecules of this type have previously been applied in the clinic for other purposes, such as acetazolamide which is used to treat glaucoma and altitude sickness. Recently, several screens of sulfonamide-containing molecules have identified several promising new candidate CA inhibitors, with inhibition coefficients (Kis) in the low nanomolar range and with good specificity for CA IX relative to other CA isoforms.[102-104] These compounds therefore can be expected to possess binding affinities to CA IX similar to those of the antibodies discussed above while exhibiting more rapid clearance.
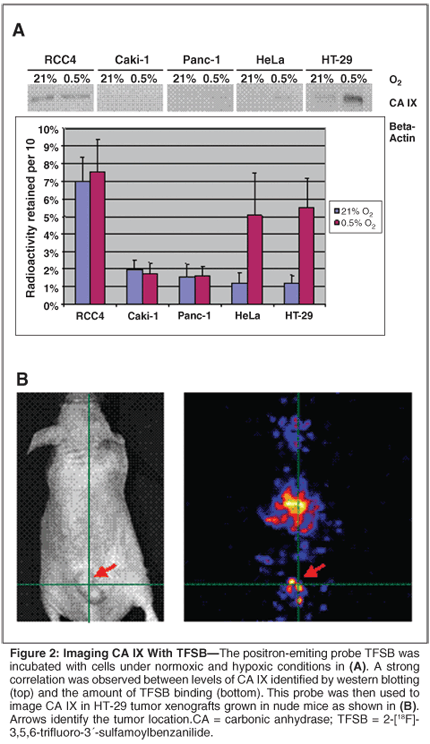
The utility of one of these small molecules as a potential imaging probe for detection of CA IX and indirectly HIF-1 activity is shown in Figure 2. The positron-emitting CA IX inhibitor 2-[18F]-3,5,6-trifluoro-3´-sulfamoylbenzanilide ([18F]-TFSB) was synthesized through an [18F]-[19F] isotope exchange reaction. Incubation of this radiotracer with cells with varying degrees of expression of CA IX demonstrated a good correlation between retention of [18F]-TFSB and the level of the protein target. Preliminary in vivo microPET imaging of this agent exhibited favorable tumor-muscle uptake ratios for CA IX-expressing HT-29 xenograft models. Background signals are evident in the area of the gut, which may be indicative of clearance pathways of TFSB and/or normal tissue expression patterns of CA IX as discussed above. Further work is currently being conducted to improve the specific activity and binding affinity and specificity of this potential clinical vehicle for imaging CA IX and HIF-1.
Role of Hypoxia Imaging in Clinical Oncology
With the emergence of sensitive, high-resolution molecular imaging modalities such as PET, single photon-emission computed tomography (SPECT), MRI/MRS, and other modalities, imaging of hypoxia and its effects is fast becoming a reality in the clinic. This rapid technologic evolution prompts rigorous assessment of the clinical utility of these practices, and careful consideration of how these techniques may best be applied toward improving outcomes in human disease while maintaining a reasonable cost-benefit ratio.
PET appears well-poised to become the dominant modality for imaging tumoral hypoxia. PET is among the most sensitive of the molecular imaging modalities, capable of detecting and quantifying radiotracer concentrations as low as nanocuries per milliliter. This allows the use of very small doses of imaging probes, alleviating concerns of toxicity associated with other types of imaging agents and chemotherapeutics that must be administered in much higher doses to be effective. Implementation of PET scanners in clinical cancer centers has accelerated in the past 10 years, with over 300 hybrid PET/computed tomography (CT) scanners installed in the United States since 2000. While fluorodeoxyglucose (FDG) remains the prevalent PET radiotracer, PET scanners are capable of detecting any positron-emitting radioisotope. Clinical management of cancer is already evolving to include PET, as FDG scans have been demonstrated to improve cancer diagnosis, staging, and therapy monitoring. This development paves the way for clinical consideration and adoption of emerging hypoxia-specific PET probes such as those described above.
Cu-ATSM and FMISO have undergone initial clinical testing and are currently being further evaluated for clinical imaging of hypoxia, specifically with regard to predicting tumor response to therapy and potentially prospectively selecting treatments based on tumor oxygenation status. Pretherapy uptake of 60Cu-ATSM has been shown to correlate with response to chemoradiotherapy in a sample of 14 cervical cancer patients.[105] Data from Rischin and colleagues have suggested that stratification of patients based on pretreatment FMISO uptake can predict tumor response to both conventional fluorouracil chemotherapy as well as hypoxia-directed tirapazamine chemotherapy.[106]
While the technology to accomplish hypoxia imaging-directed radiation therapy for the purposes of targeting radiation doses to potentially radioresistant tumor subvolumes is theoretically possible,[107-109] it remains to be seen whether this practice will result in clinically measurable improvements in patient outcome, especially as changes in oxygenation are dynamic and not static. Considering the historically quoted oxygen-enhancement ratio of 2 to 3, it is unlikely that current radiation delivery techniques will be able to boost hypoxic tumor subvolumes to the radiation doses needed to achieve cell kill comparable to aerated tissue without excessively irradiating surrounding normal tissue. It appears likely that the most effective hypoxia-directed treatments will involve a combination of local radiotherapy, conventional chemotherapy, and targeted drugs specific to hypoxic cells, HIF-1, VHL, or other hypoxia-associated targets.[34]
It is also important to prospectively assess whether measuring oxygen concentrations, hypoxia-induced physiology such as HIF-1 and CA IX, or both will be the most clinically beneficial imaging practice. Hypoxia-specific imaging methods as described above are already being introduced into the clinic, and a wealth of data suggests that oxygen concentrations are an physiologically and clinically important aspect of the tumor microenvironment. More recent interest in the biologic mechanisms by which hypoxic tumors acquire an aggressive phenotype has revealed the significance of proteins such as HIF-1 and CA IX. There is clinical evidence that the expression level of these factors can predict outcome and tumor response to therapy.[22-33]
There is also an emerging body of experimental evidence suggesting that the importance of HIF-1 and CA IX in determining tumor behavior is not solely because they are surrogates of hypoxia.[42,43,90] It has been established that these factors also exhibit oxygen-independent expression, and that this overexpression is responsible for generating some of the negative behaviors associated with hypoxic tumors. Considering this, it is apparent that these molecular entities may represent additional or complementary targets for molecular imaging beyond that of oxygen concentrations and hypoxia. Molecular imaging of HIF-1 and/or CA IX should therefore not be thought of as an alternate method of detecting hypoxia, but instead as a potentially independent useful technique for mapping important physiologic aspects of tumor cells.
Disclosures:
The authors have no significant financial interest or other relationship with the manufacturers of any products or providers of any service mentioned in this article.
References:
1. Thomlinson RH, Gray LH: The histological structure of some human lung cancers and the possible implications for radiotherapy. Br J Cancer 9:539-549, 1955.
2. Höckel M, Vaupel P: Tumor hypoxia: Definitions and current clinical, biologic, and molecular aspects. J Natl Cancer Inst 93:266-276, 2001.
3. Brown JM, Wilson WR: Exploiting tumor hypoxia in cancer treatment. Nat Rev Canc 4:437-447, 2004.
4. Read J: Mode of action of x-ray doses given with different oxygen concentrations. Br J Radiol 25:651-661, 1952.
5. Gray LH, Conger AD, Ebert M, et al: The concentration of oxygen dissolved in tissues at the time of irradiation as a factor in radiotherapy. Br J Radiol 26:638-648, 1953.
6. Wright EA, Howard-Flanders P: The influence of oxygen on the radiosensitivity of mammalian tissues. Acta Radiol 48:26-32, 1957.
7. Teicher BA, Lazo JS, Sartorelli AC: Classification of antineoplastic agents by their selective toxicities toward oxygenated and hypoxic tumor cells. Cancer Res 41:73-81, 1981.
8. Teicher BA, Holden SA, al-Achi A, et al: Classification of antineoplastic treatments by their differential toxicity toward putative oxygenated and hypoxic tumor subpopulations in vivo in the FSaII murine fibrosarcoma. Cancer Res 50:3339-3344, 1990.
9. Young SD, Marshall RS, Hill RP: Hypoxia induces DNA overreplication and enhances metastatic potential of murine tumor cells. Proc Natl Acad Sci U S A 85:9533-9537, 1988.
10. Brizel DM, Scully SP, Harrelson JM, et al: Tumor oxygenation predicts for the likelihood of distant metastases in human soft tissue sarcoma. Cancer Res 56:941-943, 1996.
11. Höckel M, Schlenger K, Aral B, et al: Association between tumor hypoxia and malignant progression in advanced cancer of the uterine cervix. Cancer Res 56:4509-4915, 1996.
12. Brizel DM, Sibley GS, Prosnitz LR, et al: Tumor hypoxia adversely affects the prognosis of carcinoma of the head and neck. Int J Radiat Oncol Biol Phys 38:285-289, 1997.
13. Cuvier C, Jang A, Hill RP: Exposure to hypoxia, glucose starvation and acidosis: effect on invasive capacity of murine tumor cells and correlation with cathepsin (L + B) secretion. Clin Exp Metastasis 15:19-25, 1997.
14. Sundfor K, Lyng H, Rofstad EK: Tumour hypoxia and vascular density as predictors of metastasis in squamous cell carcinoma of the uterine cervix. Br J Cancer 78:822-827, 1998.
15. Höckel M, Schlenger K, Höckel S, et al: Hypoxic cervical cancers with low apoptotic index are highly aggressive. Cancer Res 59:4525-4528, 1999.
16. Walenta S, Wetterling M, Lehrke M, et al: High lactate levels predict likelihood of metastases, tumor recurrence, and restricted patient survival in human cervical cancers. Cancer Res 60:916-921, 2000.
17. Wang GL, Jiang BH, Rue EA, et al: Hypoxia-inducible factor 1 is a basic-helix-loop-helix-PAS heterodimer regulated by cellular O2 tension. Proc Natl Acad Sci U S A 92:5510-5514, 1995.
18. Bruick RK, McKnight SL: A conserved family of prolyl-4-hydroxylases that modify HIF. Science 294:1337-1340, 2001.
19. Schofield CJ, Ratcliffe PJ: Oxygen sensing by HIF hydroxylases. Nat Rev Mol Cel Biol 5:343-354, 2004.
20. Chan DA, Sutphin PD, Yen SE, et al: Coordinate regulation of the oxygen-dependent degradation domains of hypoxia-inducible factor 1 alpha. Mol Cell Biol 25:6415-6426, 2005.
21. Kim W, Kaelin WG: The von Hippel-Lindau tumor suppressor protein: New insights into oxygen sensing and cancer. Curr Opin Genet Dev 13:55-60, 2003.
22. Birner P, Gatterbauer B, Oberhuber G, et al: Expression of hypoxia-inducible factor-1 alpha in oligodendrogliomas: Its impact on prognosis and neoangiogenesis. Cancer 92:165-171, 2001.
23. Korkolopoulou P, Patsouris E, Konstantinidou AE, et al: Hypoxia-inducible factor 1 alpha/vascular endothelial growth factor axis in astrocytomas. Associations with microvessel morphometry, proliferation and prognosis. Neuropathol Appl Neurobiol 30:267-278, 2004.
24. Schindl M, Schoppmann SF, Samonigg H, et al: Overexpression of hypoxia-inducible factor 1 alpha is associated with an unfavorable prognosis in lymph node-positive breast cancer. Clin Cancer Res 8:1831-1837, 2002.
25. Bos R, van der Groep P, Greijer AE, et al: Levels of hypoxia-inducible factor-1 alpha independently predict prognosis in patients with lymph node negative breast carcinoma. Cancer 97:1573-1581, 2003.
26. Dales JP, Garcia S, Meunier-Carpentier S, et al: Overexpression of hypoxia-inducible factor HIF-1 alpha predicts early relapse in breast cancer: Retrospective study in a series of 745 patients. Int J Cancer 116:734-739, 2005.
27. Birner P, Schindl M, Obermair A, et al: Overexpression of hypoxia-inducible factor 1 alpha is a marker for an unfavorable prognosis in early-stage cervical cancer. Cancer Res 60:4693-4696, 2000.
28. Burri P, Djonov V, Aebersold DM, et al: Significant correlation of hypoxia-inducible factor-1 alpha with treatment outcome in cervical cancer treated with radical radiotherapy. Int J Radiat Oncol Biol Phys 56:494-501, 2003.
29. Ishikawa H, Sakurai H, Hasegawa M, et al: Expression of hypoxia-inducible factor 1 alpha predicts metastasis-free survival after radiation therapy alone in stage IIIB cervical squamous cell carcinoma. Int J Radiat Oncol Biol Phys 60:513-521, 2004.
30. Kimura S, Kitadai Y, Tanaka S, et al: Expression of hypoxia-inducible factor (HIF)-1 alpha is associated with vascular endothelial growth factor expression and tumor angiogenesis in human oesophageal squamous cell carcinoma. Eur J Cancer 40:1904-1912, 2004.
31. Aebersold DM, Burri P, Beer KT, et al: Expression of hypoxia-inducible factor-1alpha: A novel predictive and prognostic parameter in the radiotherapy of oropharyngeal cancer. Cancer Res 61:2911-2916, 2001.
32. Koukourakis MI, Giatromanolaki A, Sivridis E, et al: Hypoxia-inducible factor (HIF1A and HIF2A), angiogenesis, and chemoradiotherapy outcome of squamous cell head-and-neck cancer. Int J Radiat Oncol Biol Phys 53:1192-1202, 2002.
33. Sivridis E, Giatromanolaki A, Gatter KC, et al: Association of hypoxia-inducible factors 1alpha and 2alpha with activated angiogenic pathways and prognosis in patients with endometrial carcinoma. Cancer 95:1055-1063, 2002.
34. Giaccia A, Siim BG, Johnson RS: HIF-1 as a target for drug development. Nat Rev Drug Discov 2:803-811, 2003.
35. Semenza GL: Targeting HIF-1 for cancer therapy. Nat Rev Cancer 3:721-732, 2003.
36. Semenza GL: Involvement of hypoxia-inducible factor 1 in human cancer. Intern Med 41:79-83, 2002.
37. Zhong H, Chiles K, Feldser D, et al: Modulation of hypoxia-inducible factor 1alpha expression by the epidermal growth factor/phosphatidylinositol 3-kinase/PTEN/AKT/FRAP pathway in human prostate cancer cells: Implications for tumor angiogenesis and therapeutics. Cancer Res 60:1541-1545, 2000.
38. Fukuda R, Hirota K, Fan F, et al: Insulin-like growth factor 1 induces hypoxia-inducible factor 1-mediated vascular endothelial growth factor expression, which is dependent on MAP kinase and phosphatidylinositol 3-kinase signaling in colon cancer cells. J Biol Chem 277:38205-38211, 2002.
39. Stiehl DP, Jelkmann W, Wenger RH, et al: Normoxic induction of the hypoxia-inducible factor-1alpha by insulin and interleukin-1beta involves the phosphatidylinositol 3-kinase pathway. FEBS Lett 512:157-162, 2002.
40. Erler JT, Bennewith KL, Nicolau M, et al: Lysyl oxidase is essential for hypoxia-induced metastasis. Nature 440:1222-1226, 2006.
41. Gatenby RA, Gillies RJ: Why do cancers have high aerobic glycolysis? Nat Rev Cancer 4:891-899, 2004.
42. Unruh A, Ressel A, Mohamed HG, et al: The hypoxia-inducible factor-1alpha is a negative factor for tumor therapy. Oncogene 22:3213-3220, 2003.
43. Williams KJ, Telfer BA, Xenaki D, et al: Enhanced response to radiotherapy in tumours deficient in the function of hypoxia-inducible factor-1. Radiother Oncol 75:89-98, 2005.
44. Kallinowski F, Zander R, Höckel M, et al: Tumor tissue oxygenation as evaluated by computerized pO2-histography. Int J Radiat Oncol Biol Phys 19:953-961, 1990.
45. Griffiths JR, Robinson SP: The OxyLite: A fibre-optic oxygen sensor. Br J Radiol 72:627-630, 1999.
46. Raleigh JA, Dewhirst MW, Thrall DE: Measuring tumor hypoxia. Semin Radiat Oncol 6:37-45, 1996.
47. Kennedy AS, Raleigh JA, Perez GM, et al: Proliferation and hypoxia in human squamous cell carcinoma of the cervix: first report of combined immunohistochemical assays. Int J Radiat Oncol Biol Phys 37:897-905, 1997.
48. Koch CJ, Evans SM, Lord EM: Oxygen dependence of cellular uptake of EF5 [2-(2-nitro-1H-imidazol-1-yl)-N-(2,2,3,3,3-pentafluoropropyl)acetamide]: Analysis of drug adducts by fluorescent antibodies vs bound radioactivity. Br J Cancer 72:869-874, 1995.
49. Zhong H, De Marzo AM, Laughner E, et al: Overexpression of hypoxia-inducible factor 1alpha in common human cancers and their metastases. Cancer Res 59:5830-5835, 1999.
50. Ivanov S, Liao S, Ivanova A, et al: Expression of hypoxia-inducible cell-surface transmembrane carbonic anhydrases in human cancer. Am J Pathol 158:905-919, 2001.
51. Hull EL, Conover DL, Foster TH: Carbogen-induced changes in rat mammary tumour oxygenation reported by near infrared spectroscopy. Br J Cancer 79:1709-1716, 1999.
52. Benaron DA, Parachikov IH, Cheong WF, et al: Design of a visible-light spectroscopy clinical tissue oximeter. J Biomed Opt 10:44005, 2005.
53. Bluestone AY, Abdoulaev G, Schmitz CH, et al: Three-dimensional optical tomography of hemodynamics in the human head. Opt Expr 9:272-286, 2001.
54. Baudelet C, Gallez B: How does blood oxygen level-dependent (BOLD) contrast correlate with oxygen partial pressure (pO2) inside tumors? Magn Reson Med 48:980-986, 2002.
55. Dunn JF, O'Hara JA, Zaim-Wadghiri Y, et al: Changes in oxygenation of intracranial tumors with carbogen: A BOLD MRI and EPR oximetry study. J Magn Reson Imaging 16:511-521, 2002.
56. Le D, Mason RP, Hunjan S, et al: Regional tumor oxygen dynamics: 19F PBSR EPI of hexafluorobenzene. Magn Reson Imaging 15:971-981, 1997.
57. Hunjan S, Mason RP, Constantinescu A, et al: Regional tumor oximetry: 19F NMR spectroscopy of hexafluorobenzene. Int J Radiat Oncol Biol Phys 41:161-171, 1998.
58. Rasey JS, Koh WJ, Grierson JR, et al: Radiolabelled fluoromisonidazole as an imaging agent for tumor hypoxia. Int J Radiat Oncol Biol Phys 17:985-991, 1989.
59. Rajendran JG, Mankoff DA, O'Sullivan F, et al: Hypoxia and glucose metabolism in malignant tumors: Evaluation by [18F]fluoromisonidazole and [18F]fluorodeoxyglucose positron emission tomography imaging. Clin Cancer Res 10:2245-2252, 2004.
60. Sorger D, Patt M, Kumar P, et al: [18F]Fluoroazomycinarabinofuranoside (18FAZA) and [18F]Fluoromisonidazole (18FMISO): a comparative study of their selective uptake in hypoxic cells and PET imaging in experimental rat tumors. Nucl Med Biol 30:317-326, 2003.
61. Reischl G, Ehrlichmann W, Bieg C, et al: Preparation of the hypoxia imaging PET tracer [18F]FAZA: reaction parameters and automation. Appl Radiat Isot 62:897-901, 2005.
62. Ziemer LS, Evans SM, Kachur AV, et al: Noninvasive imaging of tumor hypoxia in rats using the 2-nitroimidazole 18F-EF5. Eur J Nucl Med 30:259-266, 2003.
63. Lewis JS, McCarthy DW, McCarthy TJ, et al: Evaluation of 64Cu-ATSM in vitro and in vivo in a hypoxic tumor model. J Nucl Med 40:177-183, 1999.
64. Dehdashti F, Mintun MA, Lewis JS, et al: In vivo assessment of tumor hypoxia in lung cancer with 60Cu-ATSM. Eur J Nucl Med Mol Imaging 30:844-850, 2003.
65. Zanzonico P, O'Donoghue J, Chapman JD, et al: Iodine-124-labeled iodo-azomycin-galactoside imaging of tumor hypoxia in mice with serial microPET scanning. Eur J Nucl Med Mol Imaging 31:117-128, 2004.
66. Cui C, Wani MA, Wight D, et al: Reporter genes in transgenic mice. Transgenic Res 3:182-194, 1994.
67. Hadjantonakis AK, Nagy A: The color of mice: In the light of GFP-variant reporters. Histochem Cell Biol 115:49-58, 2001.
68. Yang M, Baranov E, Jiang P, et al: Whole-body optical imaging of green fluorescent protein-expressing tumors and metastases. Proc Natl Acad Sci U S A 97:1206-1211, 2000.
69. Contag CH, Spilman SD, Contag PR, et al: Visualizing gene expression in living mammals using a bioluminescent reporter. Photochem Photobiol 66:523-531, 1997.
70. Contag PR, Olomu IN, Stevenson DK, et al: Bioluminescent indicators in living mammals. Nat Med 4:245-247, 1998.
71. Bhaumik S, Gambhir SS: Optical imaging of Renilla luciferase reporter gene expression in living mice. Proc Natl Acad Sci U S A 99:377-382, 2002.
72. Gambhir SS, Bauer E, Black ME, et al: A mutant herpes simplex virus type 1 thymidine kinase reporter gene shows improved sensitivity for imaging reporter gene expression with positron emission tomography. Proc Natl Acad Sci U S A 97:2785-2790, 2000.
73. Tjuvajev JG, Doubrovin M, Akhurst T, et al: Comparison of radiolabeled nucleoside probes (FIAU, FHBG, and FHPG) for PET imaging of HSV1-tk gene expression. J Nucl Med 43:1072-1083, 2002.
74. Min JJ, Iyer M, Gambhir SS: Comparison of [18F]FHBG and [14C]FIAU for imaging of HSV1-tk reporter gene expression: Adenoviral infection vs stable transfection. Eur J Nucl Med Mol Imaging 30:1547-1560, 2003.
75. Serganova I, Doubrovin M, Vider J, et al: Molecular imaging of temporal dynamics and spatial heterogeneity of hypoxia-inducible factor-1 signal transduction activity in tumors in living mice. Cancer Res 64:6101-6108, 2004.
76. Wen B, Burgman P, Zanzonico P, et al: A preclinical model for noninvasive imaging of hypoxia-induced gene expression; comparison with an exogenous marker of tumor hypoxia. Eur J Nucl Med Mol Imaging 31:1530-1538, 2004.
77. Liu J, Qu R, Ogura M, et al: Real-time imaging of hypoxia-inducible factor-1 activity in tumor xenografts. J Radiat Res (Tokyo) 46:93-102, 2005.
78. Harada H, Kizaka-Kondoh S, Hiraoka M: Optical imaging of tumor hypoxia and evaluation of efficacy of a hypoxia-targeting drug in living animals. Mol Imaging 4:182-193, 2005.
79. Safran M, Kim WY, O'Connell F, et al: Mouse model for noninvasive imaging of HIF prolyl hydroxylase activity: assessment of an oral agent that stimulates erythropoietin production. Proc Natl Acad Sci U S A 103:105-110, 2006.
80. Cecic I, Chan DA, Sutphin PD, et al: Triple fusion reporter protein expression and activity under hypoxic conditions (abstract 171). Proceedings of the Annual Conference of the Academy of Molecular Imaging; Orlando, Florida; 2005; p 125.
81. Supuran CT, Scozzafava A, Casini A: Carbonic anhydrase inhibitors. Med Res Rev 23:146-189, 2002.
82. Hilvo M, Rafajova M, Pastoreková S, et al: Expression of carbonic anhydrase IX in mouse tissues. J Histochem Cytochem 52:1313-1322, 2004.
83. Koukourakis MI, Giatromanolaki A, Sivridis E, et al: Hypoxia-regulated carbonic anhydrase-9 (CA9) relates to poor vascularization and resistance of squamous cell head and neck cancer to chemoradiotherapy. Clin Cancer Res 7:3399-3403, 2001.
84. Loncaster JA, Harris AL, Davidson SE, et al: Carbonic anhydrase (CA IX) expression, a potential new intrinsic marker of hypoxia: correlations with tumor oxygen measurements and prognosis in locally advanced carcinoma of the cervix. Cancer Res 61:6394-6399, 2001.
85. Giatromanolaki A, Koukourakis MI, Sivridis E, et al: Expression of hypoxia-inducible carbonic anhydrase-9 relates to angiogenic pathways and independently to poor outcome in non-small cell lung cancer. Cancer Res 61:7992-7998, 2001.
86. Hui EP, Chan ATC, Pezzella F, et al: Coexpression of hypoxia-inducible factors 1alpha and 2alpha, carbonic anhydrase IX, and vascular endothelial growth factor in nasopharyngeal carcinoma and relationship to survival. Clin Cancer Res 8:2595-2604, 2002.
87. Span PN, Bussink J, Manders P, et al: Carbonic anhydrase-9 expression levels and prognosis in human breast cancer: association with treatment outcome. Br J Cancer 89:271-276, 2003.
88. Kim SJ, Rabbani ZN, Vollmer RT, et al: Carbonic anhydrase IX in early-stage non-small cell lung cancer. Clin Cancer Res 10:7925-7933, 2004.
89. Haapasalo JA, Nordfors KM, Hilvo M, et al: Expression of carbonic anhydrase IX in astrocytic tumors predicts poor prognosis. Clin Cancer Res 12:473-477, 2006.
90. Robertson N, Potter C, Harris AL: Role of carbonic anhydrase IX in human tumor cell growth, survival, and invasion. Cancer Res 64:6160-6165, 2004.
91. Oosterwijk E, Ruiter DJ, Hoedemaeker PJ, et al: Monoclonal antibody G 250 recognizes a determinant present in renal-cell carcinoma and absent from normal kidney. Int. J. Cancer 38:489-494, 1986.
92. Pastoreková S, Zavadova Z, Kostal M, et al: A novel quasi-viral agent, MaTu, is a two-component system. Virology 187:620-626, 1992.
93. Velders MP, Litvinov SV, Warnaar SO, et al: New chimeric anti-pancarcinoma monoclonal antibody with superior cytotoxicity-mediating potency. Cancer Res 54:1753-1759, 1994.
94. Zat'ovicová M, Tarábková K, Svastová E, et al: Monoclonal antibodies generated in carbonic anhydrase IX-deficient mice recognize different domains of tumour-associated hypoxia-induced carbonic anhydrase IX. J Immunol Methods 282:117-134, 2003.
95. Brouwers A, Verel I, Van Eerd J, et al: PET radioimmunoscintigraphy of renal cell cancer using 89Zr-labeled cG250 monoclonal antibody in nude rats. Cancer Biother Radiopharm 19:155-163, 2004.
96. Divgi CR, O'Donoghue JA, Welt S, et al: Phase I clinical trial with fractionated radioimmunotherapy using 131I-labeled chimeric G250 in metastatic renal cancer. J Nucl Med 45:1412-1421, 2004.
97. Brouwers AH, Dörr U, Lang O, et al: 131I-cG250 monoclonal antibody immunoscintigraphy versus [18F]FDG-PET imaging in patients with metastatic renal cell carcinoma: A comparative study. Nucl Med Commun 23:229-236, 2002.
98. Brouwers AH, Buijs WCAM, Mulders PFA, et al: Radioimmunotherapy with [131I]cG250 in patients with metastasized renal cell cancer: Dosimetric analysis and immunologic response. Clin Cancer Res 11:7178s-7186s, 2005.
99. Brouwers A, Mulders P, Oosterwijk E, et al: Pharmacokinetics and tumor targeting of 131I-labeled F(ab')2 fragments of the chimeric monoclonal antibody G250: Preclinical and clinical pilot studies. Cancer Biother Radiopharm 19:466-477, 2004.
100. Chrastina A, Závada J, Parkkila S, et al: Biodistribution and pharmacokinetics of 125I-labeled monoclonal antibody M75 specific for carbonic anhydrase IX, an intrinsic marker of hypoxia, in nude mice xenografted with human colorectal carcinoma. Int J Cancer 105:873-881, 2003.
101. Abbate F, Supuran CT, Scozzafava A, et al: Nonaromatic sulfonamide group as an ideal anchor for potent human carbonic anhydrase inhibitors: Role of hydrogen-bonding networks in ligand binding and drug design. J Med Chem 45:3583-3587, 2002.
102. Winum J, Vullo D, Casini A, et al: Carbonic anhydrase inhibitors. Inhibition of cytosolic isozymes I and II and transmembrane, tumor-associated isozyme IX with sulfamates including EMATE also acting as steroid sulfatase inhibitors. J Med Chem 46:2197-2204, 2003.
103. Vullo D, Scozzafava A, Pastoreková S, et al: Carbonic anhydrase inhibitors: inhibition of the tumor-associated isozyme IX with fluorine-containing sulfonamides. The first subnanomolar CA IX inhibitor discovered. Bioorg Med Chem Lett 14:2351-2356, 2004.
104. Cecchi A, Hulikova A, Pastorek J, et al: Carbonic anhydrase inhibitors. design of fluorescent sulfonamides as probes of tumor-associated carbonic anhydrase IX that inhibit isozyme IX-mediated acidification of hypoxic tumors. J Med Chem 48:4834-4841, 2005.
105. Dehdashti F, Grigsby PW, Mintun MA, et al: Assessing tumor hypoxia in cervical cancer by positron emission tomography with 60Cu-ATSM: relationship to therapeutic response-a preliminary report. Int J Radiat Oncol Biol Phys 55:1233-1238, 2003.
106. Rischin D, Hicks RJ, Fisher R, et al: Prognostic significance of [18F]-misonidazole positron emission tomography-detected tumor hypoxia in patients with advanced head and neck cancer randomly assigned to chemoradiation with or without tirapazamine: A substudy of Trans-Tasman Radiation Oncology Group Study 98.02. J Clin Oncol 24:2098-2104, 2006.
107. Chao KS, Bosch WR, Mutic S, et al: A novel approach to overcome hypoxic tumor resistance: Cu-ATSM-guided intensity-modulated radiation therapy. Int J Radiat Oncol Biol Phys 49:1171-1182, 2001.
108. Paulino AC, Koshy M, Howell R, et al: Comparison of CT- and FDG-PET-defined gross tumor volume in intensity-modulated radiotherapy for head-and-neck cancer. Int J Radiat Oncol Biol Phys 61:1385-1392,
2005.
109. Rajendran JG, Hendrickson KRG, Spence AM, et al: Hypoxia imaging-directed radiation treatment planning. Eur J Nucl Med Mol Imaging 33:S44-S53, 2006.
110. Höckel M, Schlenger K, Knoop C, et al: Oxygenation of carcinomas of the uterine cervix: Evaluation by computerized O2 tension measurements. Cancer Res 51:6098-6102, 1991.
111. Vaupel P, Schlenger K, Knoop C, et al: Oxygenation of human tumors: evaluation of tissue oxygen distribution in breast cancers by computerized O2 tension measurements. Cancer Res 51:3316-3322, 1991.
112. Collingridge DR, Young WK, Vojnovic B, et al: Measurement of tumor oxygenation: a comparison between polarographic needle electrodes and a time-resolved luminescence-based optical sensor. Radiat Res 147:329-334, 1997.
113. Swartz HM, Bacic G, Friedman B, et al: Measurements of pO2 in vivo, including human subjects, by electron paramagnetic resonance. Adv Exp Med Biol 361:119-128, 1994.