IMiD CC5013 Promising in Advanced Multiple Myeloma
ORLANDO-Encouraging preliminary clinical results have paved the way for phase II trials of a novel oral therapy for multiple myeloma. The study, under the leadership of Paul G. Richardson, MD, instructor in medicine, Harvard Medical School, evaluated the safety and efficacy of the immunomodulatory drug (IMiD) CC5013, a thalidomide derivative. He presented the findings at the 43rd annual meeting of the American Society of Hematology (abstract 3225).
ORLANDOEncouraging preliminary clinical results have paved the way for phase II trials of a novel oral therapy for multiple myeloma. The study, under the leadership of Paul G. Richardson, MD, instructor in medicine, Harvard Medical School, evaluated the safety and efficacy of the immunomodulatory drug (IMiD) CC5013, a thalidomide derivative. He presented the findings at the 43rd annual meeting of the American Society of Hematology (abstract 3225).
Dr. Richardson said that preclinical data showed substantial antiangiogenic activity and cytokine-related antimyeloma effects for the new agent. CC5013 directly induces apoptosis or G1 growth arrest in myeloma cell line and patient myeloma cells that are resistant to melphalan (Alkeran), doxorubicin, and dexamethasone. "In mice treated with the IMiD, there is a marked prolongation in their survival leading to eradication of the myeloma," Dr. Richardson said. "Moreover, this agent has a favorable safety profile in normal human volunteers."
The phase I dose-escalation study enrolled 26 patients at Dana-Farber Cancer Institute who had relapsed or relapsed, refractory disease. Of these patients, 65% had received prior thalidomide (Thalomid) and 60% had undergone autologous stem cell transplantation.
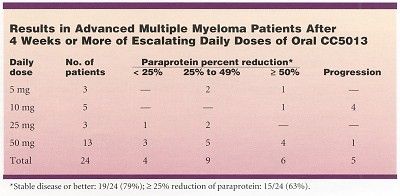
At least 3 patients were treated with daily doses of CC5013 at each of four dose levels ranging from 5 mg to 50 mg daily given for 4 weeks. Patients tolerating the drug and without disease progression were permitted to continue on therapy beyond 4 weeks as part of an extension phase for up to 1 year and beyond. When treatment extended beyond 4 weeks, dose reduction and G-CSF (Neupogen) support were permitted, Dr. Richardson said.
Median duration of treatment is currently 2 months, and, to date, 11 patients continue to receive treatment.
The results of this pilot study confirmed that CC5013 is active and well tolerated. Data showed a 25% or greater reduction in paraprotein in 15 of 24 patients (63%) who have received at least 4 weeks of therapy.
"Stable disease or better was noted in 79% of these high-risk patients who had either relapsed or relapsed and were not responding to other therapies, Dr. Richardson said. Responses were seen at lower dose levels, although the majority of responses occurred at doses higher than 5 mg/d and between 25 and 50 mg/d.
Tolerated at All Dose Levels
No dose-limiting toxicities were observed within 28 days, except for one patient in the second cohort at 10 mg/d who experienced grade 2 fever and grade 3 leukopenia and neutropenia, Dr. Richardson said. The drug was otherwise well tolerated at all dose levels during the first month of treatment.
Most patients treated with 50 mg of the drug have stabilized or responded to CC5013, but most have experienced leukopenia and also thrombocytopenia after 28 days, although no other significant attributable side effects, such as constipation, sedation, or neuropathy, have been seen.
Dr. Richardson said that phase II studies of CC5013 will begin in newly diagnosed myeloma patients and in relapsed patients, and that the agent will be studied post-transplant as a possible maintenance therapy.
"In addition to producing exciting response rates, this agent also capitalizes on convenient oral daily dosing," Dr. Richardson said. "In my opinion, this agent has considerable promise in the treatment of myeloma."
Navigating AE Management for Cellular Therapy Across Hematologic Cancers
A panel of clinical pharmacists discussed strategies for mitigating toxicities across different multiple myeloma, lymphoma, and leukemia populations.