Concurrent vs Sequential Rituximab and Fludarabine Elicits Higher Complete Response in CLL
COLUMBUS, Ohio-Concurrent administration of rituximab (Rituxan) and fludarabine (Fludara) produced higher complete response rates in previously untreated patients with chronic lymphocytic leukemia (CLL) than sequential administration, according to results of a phase II Cancer and Leukemia Group B study.
COLUMBUS, OhioConcurrent administration of rituximab (Rituxan) and fludarabine (Fludara) produced higher complete response rates in previously untreated patients with chronic lymphocytic leukemia (CLL) than sequential administration, according to results of a phase II Cancer and Leukemia Group B study.
"Hematologic toxicity on the concurrent arm is increased but manageable, with no consequences with respect to infectious morbidity," reported John C. Byrd, MD, assistant professor and director of hematologic malignancies at Ohio State University in Columbus. Stepped-up dosing, he noted, could be used to diminish the infusion toxicity that occurred in 20% of the patients receiving the concurrent treatment.
Induction Therapy
From March 1999 to January 2000, the study enrolled 104 patients53 in the sequential arm and 51 in the concurrent arm. Eligible patients had symptomatic B-cell CLL as defined by the National Cancer Institute working group, to meet the definition of required therapy, no prior therapy for CLL, and performance status of 0 to 3. The median age was 64. Modified Rai stage was 59% intermediate and 41% high risk.
Patients randomized to the sequential arm of the study received six cycles of fludarabine (25 mg/m² on days 1 to 5, every 28 days). The study design called for those in the concurrent arm to receive the same fludarabine therapy, but with rituximab (375 mg/m²) added on days 1 and 4 of cycle 1, and on day 1 only of cycles 2 to 6. Patients in both arms were observed for 2 months, assessed for induction response, and given consolidation therapy of 375 mg/m² of rituximab administered once a week for 4 weeks.
Infusion Reactions Managed
Among the first 44 patients in the concurrent arm, 9 (20%) developed grade 3 or 4 dyspnea or hypotension with the first full dose of rituximab on day 1 of treatment. "Although this resolved and usually did not occur with the second and subsequent cycles," Dr. Byrd reported, these reactions "required three patients to go off of the study and led to the hospitalization of five patients. The study was modified with stepped-up dosing." This meant giving 50 mg/m² on day 1, 325 mg/m² on day 3, and the full 375 mg/m² on day 5 for the first cycle only. With this stepped-up dosing, none of the last seven patients treated in the concurrent arm had grade 3 or 4 dyspnea or hypotension.
During the induction course of treatment, 77% of patients in the concurrent course vs 41% in the sequential course had neutropenia, a difference considered highly significant. "Thromobocytopenia and anemia were relatively similar between the two arms for induction therapy," Dr. Byrd said. Likewise, nonhematologic toxicity "was similar in the two arms, with maybe a slight increase in grade 3 to 4 dyspnea and nausea in the concurrent arm over the sequential arm," he added. Uncommon toxicities were pulmonary and neurotoxicities.
Consolidation therapy "was very well tolerated," Dr. Byrd stated. "Grade 3 or 4 neutropenia was higher in the concurrent arm37% vs 18%. Other toxicities were similar between the two arms."
Opportunistic infections numbered 8 in the concurrent arm and 14 in the sequential arm. Varicella-zoster virus accounted for 15 of these infections and was slightly more common in the sequential arm. "It must be noted," Dr. Byrd stated, "that none of the patients in this trial received prophylactic antiviral, antifungal, or Pneumocystis carinii pneumonia prophylaxis."
Significant Responses
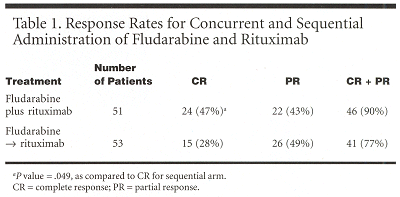
Overall response (complete plus partial response) was 90% for the concurrent arm vs 77% for the sequential arm (see Table 1). "However, the complete response was significantly higher in the concurrent arm than in the sequential arm47% vs 28%. This value was significant," Dr. Byrd said.
Follow-up is still short, with a median of 18 months, so it is difficult to assess time to progression and overall survival, but Dr. Byrd reported that patients in both arms appear to be doing well. The results of the trial, he said, warrant further testing.