Rituximab Improves Efficacy of Chemotherapy for Follicular Lymphomas
ORLANDO, Florida-Adding rituximab (Rituxan) to standard chemotherapy for follicular lymphoma regimens improves the rate and quality of responses and increases clearance of the bcl-2/IgH chimeric gene, according to studies from US and Italian cooperative groups reported at the 43rd Annual Meeting of the American Society of Hematology.
ORLANDO, FloridaAdding rituximab (Rituxan) to standard chemotherapy for follicular lymphoma regimens improves the rate and quality of responses and increases clearance of the bcl-2/IgH chimeric gene, according to studies from US and Italian cooperative groups reported at the 43rd Annual Meeting of the American Society of Hematology.
David G. Maloney, MD, PhD, reported on behalf of the Southwest Oncology Group (SWOG) that treating advanced-stage follicular lymphoma with a cycle of rituximab after standard CHOP (cyclophosphamide [Cytoxan, Neosar], doxorubicin HCl, vincristine [Oncovin], prednisone) significantly improved the quality of responses in 19% of patients, and brought the complete response rate to 54%.
Pier Luigi Zinzani, MD, PhD, reported on behalf of the Italian Cooperative Study Group on Lymphoma that adding rituximab to either FM (fludarabine [Fludara], mitoxantrone [Novantrone]) or CHOP improved both the molecular clearance rate and the quality of responses in patients with follicular lymphoma.
SWOG Study
Dr. Maloney, an associate member at the Fred Hutchinson Cancer Research Center in Seattle, reported data from the SWOG-9800 trial. "There is currently no consensus on what should be first-line therapy for advanced follicular non-Hodgkin’s lymphoma (NHL)," Dr. Maloney said. "Combination chemotherapy usually induces remissions, but nearly all patients eventually progress and there is no clear plateau on disease-free survival analysis."
Dr. Maloney reported that SWOG designed a major study of CHOP plus rituximab because CHOP produces a high response rate, and rituximab is effective in patients with nonbulky disease and has activity in relapsed disease. "Single-agent treatment with rituximab induces response rates of approximately 50% to 60% in patients with relapsed follicular NHL. In some patients, the molecular detection of disease by polymerase chain reaction (PCR) assay may be eliminated following antibody therapy," he said.
End Points Include Safety and Failure-Free Survival
The SWOG study objectives were to evaluate safety and to determine 2-year failure-free survival and the rate of disappearance of clonal bcl-2 oncogene rearrangements from the peripheral blood and bone marrow following CHOP and rituximab.
The study included 104 patients from 49 institutions. The median age was 53 years (range: 27 to 76); 54% were male; and 10% had bulky stage II, 34% stage III, and 57% stage IV disease. Thirty percent had B symptoms and 31% had bulky disease. Eighty-five were eligible for and treated with six cycles of standard CHOP (cyclophosphamide at 750 mg/m², doxorubicin at 50 mg/m², vincristine at 1.4 mg/m², and prednisone at 100 mg orally for 5 days), given at 3-week intervals. Following CHOP chemotherapy, one patient died of infection. Grade 4 toxicities included 25 hematologic events, 1 cardiovascular, 1 gastrointestinal, and 1 unknown.
Patients were restaged 4 weeks following the sixth cycle of CHOP, and those who had either a partial (PR) or complete remission (CR) were eligible for rituximab. This included 74 patients, one of whom refused treatment. The remaining 73 patients were given four doses of rituximab at 375 mg/m² each week. Following rituximab, 1 patient had grade 4 neutropenia and 12 had grade 3 toxicity.
Rituximab Improves Response; Relapses an Issue
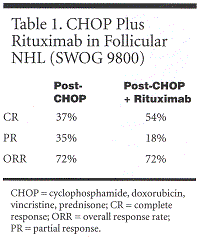
Dr. Maloney reported response data for 84 patients (see Table 1). Fifty-four percent had a confirmed or unconfirmed CR, and 18% a PR, for an overall response rate of 72%. Rituximab improved responses in 16 patients (19%), converting PR to CR in 14 patients, stable disease to CR in 1 patient, and unconfirmed CR to confirmed CR in 1 patient. The 2-year progression-free survival rate was 74%, and the 2-year overall survival rate was 95%.
"This regimen is well tolerated and overall survival is excellent, but despite the improvement in the CR rate, there continue to be relapses in these patients similar to what would be expected with chemotherapy alone," Dr. Maloney said.
To further address this problem, he said that SWOG is conducting a prospective randomized trial comparing CHOP to CHOP plus rituximab and to CHOP plus tositumomab/iodine-131 tositumomab (Bexxar) for the treatment of newly diagnosed advanced-stage follicular lymphoma.
Italian Cooperative Study Group on Lymphoma
The Italian Cooperative Study Group on Lymphoma has taken a different tack, comparing FM plus rituximab to CHOP plus rituximab as first-line treatment for follicular lymphoma, according to Dr. Zinzani, who is at the Institute of Hematology and Medical Oncology at the University of Bologna, Italy.
The 12 study centers enrolled 102 patients, 69 of whom were evaluable for response after completion of chemotherapy. All patients had histologically proven CD20-positive follicular lymphoma and a positive PCR analysis of bone marrow or peripheral blood.
Responders Eligible for Rituximab
Patients were randomized to six cycles of fludarabine at 25 mg/m²/d IV on days 1 to 5 plus mitoxantrone 10 mg/m² IV on day 1 (n = 36) or to standard CHOP (n = 33). Those in PR or CR after chemotherapy and still PCR positive were eligible for rituximab at 375 mg/m² × 4 weeks.
Dr. Zinzani said that CR rates were 64% after FM and 42% after CHOP, but that this difference was not statistically significant (P = .07). Partial response rates were 31% after FM vs 28% after CHOP.
Clearance of the bcl-2/IgH chimeric gene occurred in 34% of patients after FM and 10% of patients after CHOP (P = .007). "After rituximab there was molecular clearance in 71% of the FM patients and 44% of the CHOP patients, and there was a measurable improvement of the clinical response in 50% of PR patients," said Dr. Zinzani.