Improving the Therapeutic Ratio in Hodgkin Lymphoma Through the Use of Proton Therapy
This review addresses the rationale and evidence for-and the challenges, cost implications, and future development of-proton therapy as an important part of the treatment strategy in Hodgkin lymphoma.
The risk of serious late complications in Hodgkin lymphoma (HL) survivors has led to a variety of strategies for reducing late treatment effects from both chemotherapy and radiation therapy. With radiation therapy, efforts have included reductions in dose, reductions in the size of the target volume, and most recently, significant reductions in the dose to nontargeted normal tissues at risk for radiation damage, achieved by using the emerging technologies of intensity-modulated radiation therapy and proton therapy (PT). PT is associated with a substantial reduction in radiation dose to critical organs, such as the heart and lungs, and has the potential to improve not only the therapeutic ratio, but also both event-free and overall survival. This review addresses the rationale and evidence for-and the challenges, cost implications, and future development of-PT as an important part of the treatment strategy in HL.
Introduction
Although definitive radiotherapy (RT) and consolidative RT have been found to cure patients with Hodgkin lymphoma (HL)[1] and to improve event-free and overall survival in patients with early-stage HL treated with chemotherapy,[2] RT in HL is also responsible for some of the late toxicities that can occur 10 to 40 years following treatment. These toxicities include secondary malignancies, cardiovascular disease, hypothyroidism, cerebrovascular accidents, and muscle atrophy. Indeed, Oeffinger et al have reported a 40% cumulative incidence of grade 3 to 5 chronic toxicity attributed to chemotherapy and RT among HL survivors 25 years following treatment.[3]
RT and chemotherapy can cause agent-specific and dose-specific collateral damage to normal tissue. For example, alkylating agents cause bone marrow suppression and leukemia, while doxorubicin causes cardiac toxicity; both chemotherapy toxicities are dose-related, so strategies for reducing these risks include either completely eliminating the agents from the treatment regimen or reducing their doses. Disease control may be compromised when an agent is eliminated[4,5]; therefore, the treatment offering the highest therapeutic ratio-that is, the greatest chance of disease control with the least chance of toxicity-may involve a combination of the minimal effective doses of maximally effective agents.
Compared with chemotherapy, RT offers more potential strategies for reducing normal-tissue effects because it is a targeted therapy rather than a systemic therapy. As with chemotherapy, the dose of radiation can be reduced. But in contrast to chemotherapy, the target volume for radiation can also be reduced, eg, from total nodal irradiation to extended field irradiation (EFRT) to involved field irradiation (IFRT) to involved node irradiation (INRT).[6] Furthermore, with new radiation modalities, such as intensity-modulated radiotherapy (IMRT) and proton therapy (PT), the radiation dose inadvertently delivered to nontargeted normal tissues can also be redistributed or lowered to reduce the probabilities of particular long-term normal-tissue toxicities.
Recent attempts to eliminate RT from the initial management of HL in adults have been unsuccessful-as with the European Organisation for Research and Treatment of Cancer (EORTC)/Groupe d’tude des Lymphomes (GELA) HD10’s early closure of the chemotherapy-alone arm, reported at the 8th International Symposium on Hodgkin Lymphoma, Cologne, Germany 2010-confirming the efficacy of RT in the control of HL. In an analysis of the patterns of failure in the National Cancer Institute of Canada Clinical Trials Group/Eastern Cooperative Oncology Group (ECOG) HD6 trial-a study randomizing patients to EFRT +/− ABVD (Adriamycin [doxorubicin], bleomycin, vinblastine, dacarbazine) vs ABVD chemotherapy alone-patients who did not receive EFRT had a lower rate of disease-free survival at 12 years (87% vs 92%; P = .05) and significant increased failure rates within the expected EFRT field (20/23 vs 3/10; P = .002) and within the expected IFRT field (16/23 vs 2/10; P = .02).[7,8] Unfortunately, the long-term toxicity of the generous EFRT field led to an increase in late side effects that translated into equivalent 12-year event-free survival rates of 85% vs 80% (P = .6) and lower overall survival rates. However, late toxicities can be avoided, providing a survival advantage, because of the evolution of RT in lymphoma, which currently uses smaller radiation fields, lower radiation doses, higher-energy linear accelerators, and modern radiation techniques.
Attempts to eliminate RT from pediatric HL protocols have met with mixed success, with a number of trials showing reduced disease-free survival (DFS) in chemotherapy-alone arms. While chemotherapy-intensive trials focusing on high-risk patients have had better success in providing adequate DFS without RT, DFS has come at the cost of both acute and chronic toxicities related to the use of alkylating agents, anthracyclines, and epidophylotoxins. The greatest success has resulted from coupling an increase in dose intensity with a response-based chemotherapy approach. Similar attempts to reduce chemotherapy and radiation exposure in low-risk pediatric patients have been less successful, and there is no single accepted standard of care for patients with low-risk nodular sclerosing HL.
Normal-Tissue Radiation Dose Effects
Much of our knowledge of late radiation effects on normal tissue comes from studies of patients with HL, who typically are young at presentation, are cured with RT alone, and are long-term survivors, with a unique opportunity to develop late toxicities decades after treatment. The two most commonly reported and most critical toxicities from RT in HL survivors are secondary cancers and cardiovascular disease. In fact, these are the two leading causes of death in 10-year survivors of HL.[9]
The most common secondary malignancies in HL survivors include lung cancer, breast cancer (for women), gastrointestinal cancer, and thyroid cancer [10]; however, other rarer cancers have also been reported, such as bone sarcoma[11] and mesothelioma.[12] Over the last decade, several studies have attempted to quantify the risk of developing cancer based on the radiation dose and the use of chemotherapy. In a nested case-control study of HL survivors of at least 1 year who developed or did not develop breast cancer (1:2 match, based on registry, year of diagnosis, age at diagnosis, and follow-up time period), Travis et al[13] demonstrated that increasing the radiation dose to the breast above 4 Gy was associated with an increased risk of subsequent tumor development compared with no RT. The relative risk (RR) of 1.8 for doses of 4 to 7 Gy further increased to a RR of 8 for doses of 40 to 60 Gy, and the median time to development of a secondary breast cancer was 18 years (range, 7 to 30 years) after HL diagnosis. In a similarly designed study of HL survivors (1:2 match, based on registry, sex, year of diagnosis, age at diagnosis, and follow-up period-but not smoking status), Travis et al[14] reported an increasing risk of lung cancer with increased radiation doses to the lung of 5 Gy or more, with a median time to development of lung cancer of 10 years (range, 1 to 28 years) after HL diagnosis. The RR for developing lung cancer was 4.1 for doses of 5 to 15 Gy, but 8.6 for doses of 30 Gy or more. In a third similarly designed study, 42 survivors of HL or testicular cancer who developed gastric cancer were matched with 126 other survivors of HL or testicular cancer who did not develop gastric cancer. The study demonstrated an increased risk of gastric cancer with increasing mean stomach dose, with a median interval between RT and development of secondary stomach cancer of 15.7 years (range, 9 to 28 years). The RR for developing a subsequent gastric cancer was 9.9 for a mean stomach dose of 20 Gy or higher compared with a dose below 11 Gy.[15] Lastly, in an analysis from the Childhood Cancer Survivor Study (CCSS) evaluating the impact of radiation dose on the development of thyroid cancer, a linear relationship was seen, with an increase in the radiation dose to 20 Gy resulting in an RR of 14.6 compared with no RT. However, unlike with other organs, radiation doses greater than 20 Gy resulted in a lower risk of thyroid cancer.[6]
Along with cumulative anthracycline dose, cardiac RT has been implicated in various cardiac problems in HL survivors. Cardiomyopathy, coronary artery disease, valvular disease, and pericarditis specifically have been found to be associated with increased RT dose to the heart. Mulrooney et al[16] evaluated cardiac complications among the patients in the CCSS who were followed for a minimum of 10 years. They found an increased hazard ratio (HR) for congestive heart failure (2.2), myocardial infarction (2.4), and valvular disease (3.3) with mean cardiac doses of 15 Gy and higher compared with no radiation. Tukenova et al[17] evaluated 4,122 people who were 5-year survivors of a childhood cancer diagnosed before 1986 in France or the United Kingdom. The risk of dying as a result of cardiac diseases (n = 21) was significantly higher in patients who had received a cumulative anthracycline dose greater than 360 mg/m2 (RR, 4.4; 95% confidence interval [CI], 1.3 to 15.3) and in those who received an average radiation dose that exceeded 5 Gy to the heart (RR was 12.5 for 5 to 14.9 Gy and 25.1 for > 15 Gy).
It is therefore to be expected that the risks of these serious late radiation-related toxicities could be reduced or eliminated by further reducing the radiation dose to nontargeted critical structures, such as the heart, thyroid, breasts, and lungs.
Smaller Radiation Fields and Lower Doses
Prior to the routine use of chemotherapy, radiation fields encompassed areas known to be “involved” as well as all areas at risk for subclinical involvement. Clinical trials have assisted with the establishment of efficacious yet moderate doses of chemotherapy for control of subclinical disease, permitting a reduction in the target volume for radiation treatment from all areas at risk for subclinical disease to only the area of involvement. Over the last 20 years, the typical radiation target volume has evolved from total nodal to subtotal nodal (or EFRT) to IFRT, without compromising event-free survival or overall survival. By reducing the radiation field, the volume of nontargeted normal tissue being irradiated has also been reduced, which has translated into lower rates of toxicity.
There is substantial evidence of toxicity reduction with reduction in radiation target volume. In 1972, Stanford University began to use cardiac and subcarinal blocking after 15 Gy, which resulted in a decrease in RR for non–myocardial infarction-related cardiac death, from 5.3 to 1.4. In the HD8 study by the German Hodgkin Study Group (GHSG), patients with early-stage unfavorable HL were randomized to 4 cycles of chemotherapy followed by either EFRT or IFRT to 30 Gy (with a 10-Gy boost to bulky disease).[18] Compared with IFRT, EFRT was associated with significantly increased rates of nausea (62.5% vs 29.1%, P < .001), pharyngitis (49.1% vs 40.5%, P = .001), leucopenia (49.1 vs 33.3%, P < .001), thrombocytopenia (16.7% vs 5.5%, P < .001), and gastrointestinal toxicity (17.5% vs 4.1%, P < .001), without any significant improvement in freedom from treatment failure or overall survival for the entire cohort because of the efficacy of 4 cycles of chemotherapy in controlling subclinical disease. In a subgroup analysis of 89 patients who were 60 years old or older, World Health Organization (WHO) grade 3/4 toxicity was remarkably higher with EFRT compared with IFRT (26.5% vs 8.6%). In particular, secondary cancers, grade 3/4 leucopenia and nausea, and grade 1/2 esophagitis and pharyngitis were considerably increased in the EFRT arm.[19] Lastly, in a meta-analysis evaluating the risk of secondary cancers in randomized controlled studies of patients with HL, there was a significantly higher risk of developing breast cancer following EFRT (odds ratio [OR] = 3.25; P = .04) vs following IFRT.[20] This difference in risk is due to omission of the axillary fields in patients for whom the axilla was uninvolved, resulting in a large component of breast dose from the mantle field.
With the development of more sensitive imaging modalities (such as positron-emission tomography [PET]-computed tomography [CT]), there is greater interest in radiation fields that include only the “involved nodes.” The long-term impact of further field reduction to only an “involved-node” field has been modeled in various studies and estimated to reduce the absolute risk of a cardiac event by as much as 5.1% compared with a mantle field,[21] and to produce lower estimated RRs of breast, lung, and thyroid cancers compared with IFRT.[22]
Chemotherapy has facilitated the use of both smaller RT fields and lower radiation doses. Lower radiation doses to the target also result in lower doses to in-field nontargeted normal tissue, which should translate into less toxicity. The recent GHSG HD10 study of patients with favorable-risk stage I/II HL demonstrated that in conjunction with 2 cycles of ABVD, 20 Gy of IFRT was equivalent to 30 Gy of IFRT, with similar rates of freedom from treatment failure and overall survival. Additionally, there were more adverse events and more severe acute toxicity (grade 3/4) in patients who received 30 Gy (8.7%) than there were in those who received 20 Gy (2.9%).[23] The GHSG HD11 study of patients with unfavorable-risk stage I/II HL was a four-armed study comparing two radiation dose levels (20 Gy vs 30 Gy) and two chemotherapy regimens (4 cycles of ABVD vs BEACOPP [bleomycin, etoposide, Adriamycin, cyclophosphamide, Oncovin, procarbazine, and prednisone]).[24] Similar rates of freedom from treatment failure, overall survival, and progression-free survival were observed with 20 Gy and 30 Gy in patients receiving BEACOPP chemotherapy; however, grade 3/4 toxicity was reduced with the lower dose of RT, from 12% to 5.7%. Furthermore, 20 Gy of IFRT following 4 cycles of ABVD chemotherapy was inferior to 30 Gy of IFRT following the same chemotherapy. Thus, the efficacy of the radiation dose appears to be influenced in part by the aggressiveness of the chemotherapy regimen used. In pediatric HL, combined-modality therapy has facilitated radiation dose reduction to 15 Gy and 25 Gy, doses likely to result in significantly reduced risks of late effects from RT.
In addition to reducing the size of the radiation target and the radiation dose, using higher-energy photons and integrating advanced radiation technologies, such as IMRT and PT, can also help reduce the volume of nontargeted normal tissue exposed to radiation.
Emerging Radiation Technologies: IMRT and Proton Therapy
IMRT
IMRT is a sophisticated x-ray therapy technique that employs multiple radiation beams aimed at the target from different directions, with the beams varying in size and shape during treatment delivery to create a radiation dose distribution that is highly conformal with the 3-dimensional (3D) volume of the target. Since an x-ray beam deposits radiation throughout its entire path, areas where the radiation beams intersect receive high doses, while areas traversed by only one beam receive much lower doses. With the increased number of beams, the volume of tissue receiving the highest doses conforms more precisely to the actual target volume than is the case with simpler conventional RT techniques. Because of the increased number of beams used, however, a larger volume of nontargeted tissue receives some radiation dose compared with the simpler conventional RT techniques. In essence, IMRT redistributes the radiation dose to nontargeted tissues in a way that can be favorable to tissues at risk for a particular toxicity. Nonetheless, with both conventional and IMRT x-ray–based techniques, most of the total radiation dose is actually deposited outside the target.
FIGURE
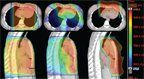
Color-Wash Dose Distribution for Three Plans for a Representative Patient With Mediastinal Involvement of Hodgkin Lymphoma
Several published studies have compared the dose distributions of conventional 3D conformal RT (3DCRT) plans (typically designed as anterior-posterior/posterior-anterior [AP/PA] plans) with IMRT plans in patients with HL. In one of the first studies, investigators from Memorial Sloan-Kettering Cancer Center compared IMRT vs 3DCRT (AP/PA) and demonstrated a 12% reduction in mean lung dose with IMRT.[25] In another study, by Girinsky et al, IMRT was better able to protect the heart and coronary arteries than was 3DCRT, but with IMRT there was more concern regarding increased volume of normal tissue receiving “low doses” of RT than there was with 3DCRT.[26] More recently, Weber et al reported that in a nonlinear model of the development of secondary malignancies, IMRT increased the risk of breast, lung, and thyroid cancers compared with 3DCRT, in part because of the redistribution of radiation dose with IMRT that leads to an increased volume of normal tissue-which would not have been irradiated at all with 3DCRT-receiving “low doses” of RT.[22] Similarly, a recent study from the GHSG[27] demonstrated reduced dose to the heart and spinal cord with the use of IMRT, but increased dose to the lungs and breasts compared with 3DCRT.
Only one study has reported on outcomes in HL patients treated with IMRT.[28] Paumier et al reported on 32 patients treated to an INRT field with IMRT following chemotherapy and demonstrated 5-year progression-free survival and overall survival of 91% and 95%, respectively, comparable to what is achieved with standard techniques. Only one patient developed an in-field relapse, and one developed grade 3 pneumonitis.
Protons
Unlike x-rays, protons are charged particles with mass. Protons travel a finite distance, determined by their acceleration and the composition of the matter through which they travel; thus, the actual range of protons in tissue can be controlled, thereby eliminating the “exit” dose to nontargeted tissues. In addition, protons deposit most of their radiation dose in tissue near the end of their range in a striking pattern called the Bragg peak, with relatively little dose deposited along the “entrance” path. Whereas with the IMRT technique radiation dose to nontargeted tissue is redistributed, with PT it can actually be significantly reduced.
TABLE 1

Toxicity and Critical Doses to Structures by Treatment Technique in Patients With Mediastinal Hodgkin Lymphoma
PT reduces high-, medium-, and low-dose radiation levels compared with 3DCRT. Dosimetric studies evaluating the use of PT in HL date back to 1974, when Archambeau et al explored the use of PT for total nodal irradiation.[29] In that study, the investigators demonstrated that PT could reduce the irradiated volume by 50% compared with photons. More sophisticated treatment planning studies have since been published. In a prospective phase II study of involved-node radiotherapy in patients with mediastinal HL,[30] the first 10 patients enrolled underwent treatment planning with 3DCRT (AP/PA), IMRT, and PT and were offered treatment with the plan that best spared the organs at risk while maintaining appropriate target coverage. In all 10 cases, PT was associated with the best plan and all patients were offered treatment with PT. The Figure shows the color-wash isodose distributions for the 3DCRT, IMRT, and PT plans for one of these patients. The Table describes the expected dose-volume effects from EFRT (mantle radiation) using 3DCRT, from IFRT using 3DCRT, from INRT using 3DCRT, from IMRT, and from PT; these data demonstrate the considerable dose reductions achieved with each successive treatment approach.
Organ-Specific Radiation Dose Reduction With PT
Using modern RT techniques for the treatment of HL, radiation oncologists must balance the risks and benefits of RT pathways through the various organs involved. Although secondary cancers are considered the biggest RT-associated concern for HL survivors, RT toxicity to the heart is responsible for more deaths than any other specific organ malignancy. Thus, at the University of Florida (UF), we attempt to limit the dose to all nontargeted normal tissue, but we prioritize the organs at risk in the following order: heart, lungs, breasts (women), and esophagus.
Heart
In a prospective UF study of 10 patients with HL who received 30–39.6 Gy of INRT,[30] the mean dose to the heart was 19.4 Gy with 3DCRT, 12.2 Gy with IMRT, and 8.9 Gy (relative biological effectiveness [RBE]) with PT. With PT, the dose was reduced by > 5 Gy in 9 of the 10 patients compared with 3DCRT, and in 4 of the 10 patients compared with IMRT. In a study from MD Anderson Cancer Center (MDACC) of 10 patients with mediastinal lymphoma, PT similarly reduced the mean heart dose by 9 Gy compared with 3DCRT.[31] In a follow-up UF study evaluating the dose to the subunits of the heart,[32] the mean doses with 3DCRT, IMRT, and PT, respectively, were: left ventricle, 13 Gy, 5 Gy, and 0 Gy (RBE); right ventricle, 17 Gy, 11 Gy, and 9 Gy (RBE); left atrium, 28 Gy, 15 Gy, and 5 Gy (RBE); right atrium, 24 Gy, 17 Gy, and 11 Gy (RBE); mitral valve, 28 Gy, 9 Gy, and 0 Gy (RBE); tricuspid valve, 19 Gy, 13 Gy, and 0 Gy (RBE); aortic valve, 30 Gy, 18 Gy, and 9 Gy (RBE); left circumflex artery, 30 Gy, 16 Gy, and 5 Gy (RBE), and left anterior descending artery, 18 Gy, 10 Gy, and 5 Gy (RBE).
Lungs
In the UF study,[30] mean lung dose was 13.2 Gy for 3DCRT, 10.6 Gy for IMRT, and 7.1 Gy (RBE) for PT. In particular, PT reduced the dose to the lungs by > 5 Gy in six patients compared with 3DCRT and in two patients compared with IMRT. Similarly, MDACC demonstrated a reduction in mean lung dose of 3.3 Gy compared with 3DCRT.[31] Because of tissue density in the lungs, however, the doses for the PT plans might have been slightly underestimated.
Breasts
In the UF study,[30] the mean breast dose was not substantially reduced because of the limited treatment field, which resulted in mean breast doses that averaged 5.4 Gy for 3DCRT, 5.5 Gy for IMRT, and 4.6 Gy for PT. However, in two patients with residual bulky disease extending posterior to the breast, PT was able to reduce the mean breast dose by > 5 Gy compared with 3DCRT or IMRT. In the MDACC study, only a marginal benefit was seen in the mean dose to the breasts with PT compared with 3DCRT (5.9 Gy vs 6.1 Gy).[31] In a study by Andolino et al, however, PT reduced the mean dose to the breasts to 1 Gy-from 4.7 Gy with 3DCRT.[33] Because this study chose a posterior approach, with the beam entering the anterior mediastinum through the posterior chest and heart, the heart received a higher mean dose with PT (17 Gy) than with 3DCRT (14 Gy), highlighting the occasional tradeoff with central tumors of sparing anterior vs posterior structures. In a case report of a posterior mediastinal HL, the breast dose was reduced with PT from 5.7 Gy to 1.7 Gy, and the mean heart dose was reduced from 24 Gy to 11 Gy.[34]
Esophagus
In the UF study, the mean esophagus dose was 22.6 Gy for 3DCRT, 17.2 Gy for IMRT, and 17 Gy for PT. PT reduced the dose by > 5 Gy in 7 of the 10 patients compared with 3DCRT and in 3 patients compared with IMRT. In the MDACC study, PT reduced the dose to the esophagus, on average, by 13 Gy compared with 3DCRT.[31]
Other structures
PT can also reduce the dose to other important structures in patients with HL. In a case report of a prepubescent pediatric patient with HL, PT was able to reduce the number of thoracic vertebral bodies irradiated by five, which should result in less of an impact on patient growth.[35] Lastly, subdiaphragmatic HL is treated with target volumes and fields similar to those used for seminoma (para-aortic fields +/− pelvic fields); two studies have demonstrated that PT could reduce the dose to the stomach, bowel, bladder, and ipsilateral kidney, compared with 3DCRT and IMRT.[36,37]
Uncertainties
PT treatment planning is more complex than x-ray treatment planning. The depth that protons travel in tissue depends on their energy and the composition of their pathway. Minor variations in daily patient positioning may result in minor variations in the proton path length, which must be accounted for in the treatment planning process. Improved treatment planning and delivery systems will reduce this uncertainty and minimize adjustments needed in the treatment planning process, leading to even more conformal PT dose distributions in the future. While concerns have been raised about uncertainty related to secondary neutron scatter in patients receiving double-scatter PT, these effects are minimal when considered in the context of the reduction in dose achieved with PT vs x-ray treatment. Clinical experience suggests no increase in the risk of second malignancy with PT, meaning that the impact of neutrons has been immeasurably small.[38]
Financial Costs
As is the case with most medical advances, PT is more expensive than IMRT or 3DCRT. According to the Centers for Medicare and Medicaid fee schedule for local 99, reimbursements for a patient receiving 30.6 Gy at 1.8 Gy per fraction with 3DCRT, IMRT, and PT are approximately $6,000, $15,000, and $23,000, respectively. However, considering the CCSS report indicating that 40% of HL survivors have a grade 3 or higher chronic health condition 25 years following treatment, a reduction in these chronic health problems by even a fraction through the use of PT may result in a lessening of the cost discrepancy over time.[3]
Guidelines
Although clinical studies of HL have been published by investigators from UF and MDACC, a randomized study would be impossible due to the low incidence of HL in the population and the decades of follow-up needed to quantify a difference in late toxicity. The most recent National Compre-hensive Cancer Network (NCCN) guidelines (January 2012) stipulate that treatment with either photons or protons is acceptable. Moreover, upcoming pediatric HL studies being developed by the Children’s Oncology Group will consider including PT to help reduce long-term treatment toxicity.
In summary, advances in the treatment of HL over the last 30 years have dramatically reduced the RT dose to nontargeted normal tissue. Some of these advances, such as IFRT, are only now being fully appreciated, with recent studies demonstrating less long-term toxicity. However, many of the more recent technological developments, including INRT, IMRT, and PT, are unlikely to demonstrate improvements in toxicity for at least another decade. Despite these limitations, modern techniques should be used in future clinical studies in an attempt to reduce late chronic morbidity.
Financial Disclosure:Dr. Li is a member of the Speakers Bureau for IBA (Ion Beam Applications). The rest of the authors have no significant financial interest or other relationship with the manufacturers of any products or providers of any service mentioned in this article.
Acknowledgement:The authors would like to thank Jessica Kirwan for her help with preparation of the manuscript.
References:
References
1. Mendenhall NP, Rodrigue LL, Moore-Higgs GJ, et al. The optimal dose of radiation in Hodgkin’s disease: an analysis of clinical and treatment factors affecting in-field disease control. Int J Radiat Oncol Biol Phys. 1999;44:551-61.
2. Thomas J, Ferme C, Noordijk EM, et al. Six cycles of ABVD + IF-RT vs four cycles of ABVD + IF-RT vs four cycles of BEACOPP + IF-RT in unfavourable supradiaphragmatic clinical stages I-II Hodgkin’s lymphoma: the EORTC-GELA H9-U randomized clinical trial (20982) in 808 patients. Eur J Haematol. 2004;73: A-E12, 40.
3. Oeffinger KC, Mertens AC, Sklar CA, et al. Chronic health conditions in adult survivors of childhood cancer. N Engl J Med. 2006;355:1572-82.
4. Straus DJ, Johnson JL, LaCasce AS, et al. Doxorubicin, vinblastine, and gemcitabine (CALGB 50203) for stage I/II nonbulky Hodgkin lymphoma: pretreatment prognostic factors and interim PET. Blood. 2011;117:5314-20.
5. Borchmann P, Diehl V, Goergen H, et al. Dacarbazine is an essential component of ABVD in the treatment of early favourable Hodgkin lymphoma: results of the second interim analysis of the GHSG HD13 Trial. Haematologica. 2010;95:473.
6. Bhatti P, Veiga LH, Ronckers CM, et al. Risk of second primary thyroid cancer after radiotherapy for a childhood cancer in a large cohort study: an update from the childhood cancer survivor study. Radiat Res. 2010;174:741-52.
7. Macdonald DA, Ding K, Gospodarowicz MK, et al. Patterns of disease progression and outcomes in a randomized trial testing ABVD alone for patients with limited-stage Hodgkin lymphoma. Ann Oncol. 2007; 18:1680-4.
8. Meyer RM, Gospodarowicz MK, Connors JM, et al. ABVD alone versus radiation-based therapy in limited-stage Hodgkin’s lymphoma. N Engl J Med. 2012;
366:399-408.
9. Ng AK, Bernardo MP, Weller E, et al. Long-term survival and competing causes of death in patients with early-stage Hodgkin’s disease treated at age 50 or younger. J Clin Oncol. 2002;20:2101-8.
10. Hodgson DC, Gilbert ES, Dores GM, et al. Long-term solid cancer risk among 5-year survivors of Hodgkin’s lymphoma. J Clin Oncol. 2007;25:1489-97.
11. Smith J. Postradiation sarcoma of bone in Hodgkin disease. Skeletal Radiol. 1987;16:524-32.
12. De Bruin ML, Burgers JA, Baas P, et al. Malignant mesothelioma after radiation treatment for Hodgkin lymphoma. Blood. 2009;113:3679-81.
13. Travis LB, Hill DA, Dores GM, et al. Breast cancer following radiotherapy and chemotherapy among young women with Hodgkin disease. JAMA. 2003;
290:465-75.
14. Travis LB, Gospodarowicz M, Curtis RE, et al. Lung cancer following chemotherapy and radiotherapy for Hodgkin’s disease. J Natl Cancer Inst. 2002;94:182-92.
15. van den Belt-Dusebout AW, Aleman BM, Besseling G, et al. Roles of radiation dose and chemotherapy in the etiology of stomach cancer as a second malignancy. Int J Radiat Oncol Biol Phys. 2009;75:1420-9.
16. Mulrooney DA, Yeazel MW, Kawashima T, et al. Cardiac outcomes in a cohort of adult survivors of childhood and adolescent cancer: retrospective analysis of the Childhood Cancer Survivor Study cohort. BMJ. 2009;339:b4606.
17. Tukenova M, Guibout C, Oberlin O, et al. Role of cancer treatment in long-term overall and cardiovascular mortality after childhood cancer. J Clin Oncol. 2010;28:1308-15.
18. Engert A, Schiller P, Josting A, et al. Involved-field radiotherapy is equally effective and less toxic compared with extended-field radiotherapy after four cycles of chemotherapy in patients with early-stage unfavorable Hodgkin’s lymphoma: results of the HD8 trial of the German Hodgkin’s Lymphoma Study Group. J Clin Oncol. 2003;21:3601-8.
19. Klimm B, Eich HT, Haverkamp H, et al. Poorer outcome of elderly patients treated with extended-field radiotherapy compared with involved-field radiotherapy after chemotherapy for Hodgkin’s lymphoma: an analysis from the German Hodgkin Study Group. Ann Oncol. 2007;18:357-63.
20. Franklin J, Pluetschow A, Paus M, et al. Second malignancy risk associated with treatment of Hodgkin’s lymphoma: meta-analysis of the randomised trials. Ann Oncol. 2006;17:1749-60.
21. Maraldo MV, Brodin NP, Vogelius IR, et al. Risk of developing cardiovascular disease after involved node radiotherapy versus mantle field for Hodgkin lymphoma. Int J Radiat Oncol Biol Phys. 2012 Jan 21. [Epub ahead of print]
22. Weber DC, Johanson S, Peguret N, et al. Predicted risk of radiation-induced cancers after involved field and involved node radiotherapy with or without intensity modulation for early-stage Hodgkin lymphoma in female patients. Int J Radiat Oncol Biol Phys. 2011;
81:490-7.
23. Engert A, Plutschow A, Eich HT, et al. Reduced treatment intensity in patients with early-stage Hodgkin’s lymphoma. N Engl J Med. 2010;363:640-52.
24. Eich HT, Diehl V, Gorgen H, et al. Intensified chemotherapy and dose-reduced involved-field radiotherapy in patients with early unfavorable Hodgkin’s lymphoma: final analysis of the German Hodgkin Study Group HD11 trial. J Clin Oncol. 2010;28:4199-206.
25. Goodman KA, Toner S, Hunt M, et al. Intensity-modulated radiotherapy for lymphoma involving the mediastinum. Int J Radiat Oncol Biol Phys. 2005;
62:198-206.
26. Girinsky T, Pichenot C, Beaudre A, et al. Is intensity-modulated radiotherapy better than conventional radiation treatment and three-dimensional conformal radiotherapy for mediastinal masses in patients with Hodgkin's disease, and is there a role for beam orientation optimization and dose constraints assigned to virtual volumes? Int J Radiat Oncol Biol Phys. 2006;64:218-26.
27. Koeck J, Abo-Madyan Y, Lohr F, et al. Radiotherapy for early mediastinal Hodgkin lymphoma according to the German Hodgkin Study Group (GHSG): the roles of intensity-modulated radiotherapy and involved-node radiotherapy. Int J Radiat Oncol Biol Phys. 2012;83:
268-76.
28. Paumier A, Ghalibafian M, Beaudre A, et al. Involved-node radiotherapy and modern radiation treatment techniques in patients with Hodgkin lymphoma. Int J Radiat Oncol Biol Phys. 2011;80:199-205.
29. Archambeau JO, Bennett GW, Levine GS, et al. Proton radiation therapy. Radiology. 1974;110:445-57.
30. Hoppe BS, Flampouri S, Su Z, et al. Consolidative involved-node proton therapy for stage IA-IIIB mediastinal Hodgkin lymphoma: preliminary dosimetric outcomes from a phase II study. Int J Radiat Oncol Biol Phys. 2012;83:260-7.
31. Li J, Dabaja B, Reed V, et al. Rationale for and preliminary results of proton beam therapy for mediastinal lymphoma. Int J Radiat Oncol Biol Phys. 2011;81:167-74.
32. Hoppe BS, Flampouri S, Su Z, et al. Effective dose reduction to cardiac structures using protons compared with 3DCRT and IMRT in mediastinal Hodgkin lymphoma. Int J Radiat Oncol Biol Phys. 2012; in press.
33. Andolino DL, Hoene T, Xiao L, et al. Dosimetric comparison of involved-field three-dimensional conformal photon radiotherapy and breast-sparing proton therapy for the treatment of Hodgkin’s lymphoma in female pediatric patients. Int J Radiat Oncol Biol Phys. 2011;81:e667-71.
34. Hoppe BS, Flampouri S, Li Z, Mendenhall NP. Cardiac sparing with proton therapy in consolidative radiation therapy for Hodgkin lymphoma. Leuk Lymphoma. 2010;51:1559-62.
35. Figura NB, Flampouri S, Hopper K, et al. Consolidative proton therapy following high-dose chemotherapy and autologous stem cell transplant in an adolescent with relapsed Hodgkin lymphoma.
J Adolesc Young Adult Oncol. 2011;1:103-06.
36. Simone CB, 2nd, Kramer K, O’Meara WP, et al. Predicted rates of secondary malignancies from proton versus photon radiation therapy for stage I seminoma. Int J Radiat Oncol Biol Phys. 2012;82:242-9.
37. Hoppe BS, Mamalui-Hunter M, Mendenhall NP, et al. Improving the therapeutic ratio by using proton therapy in patients with stage I or II seminoma. Am J Clin Oncol. 2011 Nov 29. [Epub ahead of print]
38. Chung CS, Keating N, Yock T, Tarbell NJ. Comparative analysis of second malignancy risk in patients treated with proton therapy versus conventional photon therapy. Int J Radiat Oncol Biol Phys. 2008;72:S8.