Incidence and Management of Renal Adverse Events in Patients With Relapsed and/or Refractory Multiple Myeloma Treated With Single-Agent Carfilzomib
This article reviews the etiology and incidence of renal adverse events in patients with multiple myeloma, the renal safety profile of single-agent carfilzomib from four phase II studies in patients with relapsed and/or refractory multiple myeloma, and the management of patients with multiple myeloma who receive carfilzomib and are at risk for renal complications.
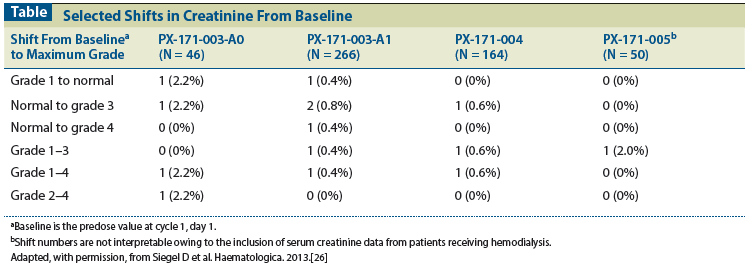
Table: Selected Shifts in Creatinine From Baseline
Patients with multiple myeloma (MM) frequently experience renal dysfunction owing to patient-specific risk factors, the pathophysiology of MM, and treatment-related adverse events. The presence of renal complications in patients with MM may be associated with advanced disease and is a negative prognostic factor for survival. Frequently these patients receive reduced or modified dosing regimens, which can result in under-dosing and may adversely affect treatment efficacy. Consequently, there is a need for effective therapies with favorable renal safety profiles. Carfilzomib is a selective proteasome inhibitor approved in the United States as a single agent for the treatment of relapsed and refractory MM. Safety studies have demonstrated that single-agent carfilzomib is well tolerated in patients with relapsed and/or refractory MM and concomitant renal dysfunction. This article reviews the etiology and incidence of renal adverse events in patients with MM, the renal safety profile of single-agent carfilzomib from four phase II studies in patients with relapsed and/or refractory MM, and the management of patients with MM who receive carfilzomib and are at risk for renal complications.
Introduction
The treatment of patients with multiple myeloma (MM) is frequently complicated by the presence of comorbid conditions at the time of diagnosis and by the onset of treatment-induced adverse events (AEs). The etiology of these comorbidities and complications is multifactorial, with contributions from patient-specific factors, MM pathophysiology, and treatment-related AEs. Patients with MM are typically older (75%–79% are > 70 years old)[1] and are therefore more likely to have pre-existing conditions, such as diabetes, hypertension, hyperlipidemia, peripheral vascular disease, cardiac disease, and chronic kidney disease. Uncontrolled proliferation of plasma cells in patients with MM can result in myelosuppression, immunosuppression, and end-organ damage (including bone lesions, hypercalcemia, anemia, and renal insufficiency).[2] Although efficacious, anti-myeloma treatments can also be associated with substantial AEs, including hematologic, neuropathic, cardiac, pulmonary, and renal complications.
Renal insufficiency is defined as an elevated serum creatinine level and decreased creatinine clearance (CrCl) or glomerular filtration rate (GFR). It is a common comorbidity in patients with MM. Up to 50% of patients present with a decrease in CrCl at the time of diagnosis of MM, and renal insufficiency or progressive renal failure develops in approximately 20% to 60% of patients during the course of the disease.[3-5] An increased risk of chronic renal disease has been linked to patient-specific risk factors, such as race or ethnicity (African Americans, Hispanics, and American Indians are at greater risk), family history of chronic kidney disease, and age older than 60 years.[6,7] In addition, comorbidities such as diabetes or cardiovascular disease (including hypertension, hypercholesterolemia, and coronary artery disease), which are frequently seen in the elderly, are associated with an increased risk of kidney damage.[6,7]
In patients with MM, the pathogenesis of renal dysfunction is associated with the toxic effects of excess monoclonal light chain production. Deposition of light chains in tissues can lead to amyloid light chain amyloidosis and light chain deposition disease, which frequently develop into nephrotic-range proteinuria and renal failure. In addition, the production of excess light chains can overwhelm the filtration capacity of the renal tubules; this can lead to the formation of casts in the tubules, with consequent renal obstruction.[3,5]
The treatment of MM may also lead to renal dysfunction, owing to the sensitivity of the organ to injury from tumor lysis syndrome or intravenous contrast dyes used for positron emission tomography (PET) scans and CT scans, which can aggravate damage caused by light chains and/or promote aggregate formation.[3] Pre-renal azotemia is also frequently observed in patients with MM and can result from volume depletion due to hypercalcemia, congestive heart failure, or treatment-related AEs (eg, nausea, vomiting, diarrhea, and fever).
Renal failure and renal insufficiency have been shown to affect the prognosis of patients with MM.[8-10] Multivariate analyses have identified an estimated GFR of < 30 mL/min/1.73 m2[10] or a plasma creatinine level of ≥ 1.5 mg/dL[8] as significant negative prognostic factors for overall survival.
Although patients with renal failure typically have a higher tumor burden and more aggressive disease,[9] they often require reduced or modified dosing regimens with anti-myeloma treatments, particularly with agents that are dependent on renal clearance and/or have a pharmacokinetic profile that is influenced by the degree of renal dysfunction.[9,11,12] Melphalan, for instance, is both secreted and reabsorbed by renal tubules, and it can be toxic in patients with renal impairment.[13] Therefore, dose reductions of melphalan are needed in the setting of renal insufficiency.[13,14] The immunomodulatory drug (IMiD) lenalidomide is similarly dependent on renal clearance, and in phase II and III studies increased incidences of thrombocytopenia and grade ≥ 3 myelosuppression were reported in patients with severe renal impairment treated with lenalidomide and dexamethasone.[15,16] As with melphalan, lenalidomide dosing must be reduced for patients with moderate or severe renal impairment and for those receiving dialysis (CrCl ≤ 60 mL/min).[17] Although the most recently approved IMiD, pomalidomide, and its metabolites are primarily excreted through the kidney, only limited data are available on the incidence of renal toxicity following its use or on what, if any, pomalidomide-related systemic toxicities are associated with impaired renal function.[18] Other drugs such as anthracyclines, corticosteroids (including dexamethasone), the alkylating agent cyclophosphamide, the IMiD thalidomide, and the proteasome inhibitor bortezomib do not require dose reductions in patients with renal dysfunction, probably because of their low rates of clearance by the kidneys and/or the insensitivity of their pharmacokinetic profiles to the level of renal impairment.[11,14,19,20]
The selective proteasome inhibitor carfilzomib received accelerated approval in the United States in 2012 for single-agent use in patients with relapsed and refractory MM. In the pivotal phase II trial PX-171-003-A1, single-agent carfilzomib was well tolerated and exhibited durable activity in patients with relapsed and refractory MM (N = 257), with an overall response rate of 22.9%, median duration of response of 7.8 months, and median overall survival of 15.4 months.[21] Clinical studies have shown that the kidneys are not a major pathway for carfilzomib clearance, because < 1% of the total administered dose is recoverable from urine samples 24 hours after dosing.[22] Below, I review the renal safety profile of single-agent carfilzomib in more than 500 patients with relapsed and/or refractory MM from four phase II trials, and I offer recommendations for the management of patients with renal impairment and those who experience renal AEs.
Cross-Trial Safety Analysis of Phase II Carfilzomib Trials: Key Renal AEs
A cross-trial safety analysis examined renal AEs in the phase II trials PX-171-003-A0, PX-171-003-A1, PX-171-004, and PX-171-005,[21-25] totaling 526 patients with relapsed and/or refractory MM who were treated with single-agent carfilzomib.[26] The safety data from these four trials, along with efficacy data from the PX-171-003-A1 trial, supported the US Food and Drug Administration’s accelerated approval of carfilzomib.[27] The PX-171-005 trial enrolled patients with various degrees of renal function, ranging from normal function to dysfunction that necessitated long-term dialysis,[22] while the other three trials required that patients have a serum creatinine level of < 2 mg/dL and an estimated GFR of > 30 mL/min/1.73 m2.
Patients were heavily pretreated, with a median of four previous regimens.[26] At baseline, 63.2% of patients had mild to severe renal dysfunction (39.4% with a CrCl of ≥ 50 to < 80 mL/min and 23.8% with a CrCl of < 50 mL/min; in this latter category, 4.0% of all patients had a CrCl of < 30 mL/min, including eight patients receiving dialysis). Importantly, patients with renal dysfunction at baseline, including those undergoing dialysis, were able to receive the same carfilzomib doses as those with normal renal function and, despite the high percentage of patients with baseline renal dysfunction, grade 3/4 renal AEs were uncommon overall (7.2%). Approximately one-third (33.1%) of all patients experienced at least one grouped renal impairment AE of any grade, of which nearly one-half (48%) were attributable to disease progression. The most common acute renal failure (ARF) Standardized MedDRA Query AEs were increased blood creatinine (24.1%), ARF (5.3%), renal failure (3.8%), increased blood urea (2.7%), and decreased CrCl (1.1%). Among patients who experienced renal AEs, 10.9% required dose reductions, 12.1% discontinued single-agent carfilzomib treatment, 23.6% had missed doses, and 3.4% had delayed doses. The remaining 50% of patients who reported renal AEs were able to maintain their current carfilzomib therapy.
In patients treated with single-agent carfilzomib, worsening of renal function (defined as a doubling or more of serum creatinine level from baseline) was uncommon; 13% (n = 68) of patients with evaluable creatinine levels had worsening renal function. Importantly, among patients who experienced worsening renal function, the effect was transient (ie, the serum creatinine level returned to within 20% of baseline) in almost one-half of patients (31/68), with a median duration of 1.4 weeks and a median of one episode per patient. Of the 31 patients with transient worsening renal function, 16 discontinued carfilzomib treatment, the majority because of disease progression; none discontinued as a result of a renal AE. Among the 15 patients who continued treatment, 12 recovered renal function while receiving their original dose of carfilzomib and 3 recovered renal function while receiving a reduced dose. Of the 37 patients who experienced nontransient worsening of renal function, 8 discontinued carfilzomib treatment because of an AE related to renal dysfunction. The incidence of first episodes of worsening renal function was evenly distributed across treatment cycles with a median time to first episode of 44.5 days (cycle 2), indicating a lack of cumulative toxicity. Overall, shifts of serum creatinine levels from normal or grade 1 to grade 3/4 were rare (2.3%) (Table).
Analyses performed specifically in trial PX-171-005 (N = 50) demonstrated that carfilzomib pharmacokinetics and safety were unaffected by the degree of baseline renal dysfunction. Patients were classified according to CrCl into the following groups: normal function (CrCl > 80 mL/min; n = 12), mild impairment (CrCl 50–80 mL/min; n = 12), moderate impairment (CrCl 30–49 mL/min; n = 10), severe impairment (CrCl < 30 mL/min; n = 8), and long-term dialysis (n = 8). Carfilzomib clearance, peak plasma concentration, and total exposure did not vary significantly among these groups. The carfilzomib dose was safely escalated from 15 mg/m2 to 27 mg/m2 in patients with impaired renal function in the PX-171-005 trial, and dose escalation did not appear to be associated with clinically relevant nephrotoxicity.[22] Furthermore, the frequency and severity of AEs were similar among patients with various degrees of baseline renal dysfunction, including those receiving hemodialysis.[22]
Clinician Perspective
A significant proportion of patients with MM have or will develop renal insufficiency at some point during the progression of the disease, as a result of patient factors, underlying disorders, or treatment-related toxicities. In the four phase II carfilzomib trials summarized here, ARF was reported for 5.3% of patients treated with single-agent carfilzomib. Discontinuation of treatment owing to a grouped renal AE was reported in 4% of carfilzomib-treated patients, a rate similar to that previously reported with single-agent lenalidomide treatment (3%).[28] It is important to note that the degree to which anti-MM treatment contributed to worsening renal function is difficult to assess in single-arm trials such as these, especially given the detrimental effect of progressive MM itself on renal function, and, in the case of carfilzomib, the heavily pretreated/refractory patients and high rate of baseline renal insufficiency present in patients included in the cross-trial safety analysis.
In the setting of ARF, rapid intervention is critical because chronic renal insufficiency can adversely affect quality of life and overall survival. Plasma exchange or free light chain removal through hemodialysis that uses dialyzers with high-molecular-weight membranes may be beneficial in treating ARF, although their use is still controversial and the procedures may not be available at many community hospitals.[3,17] Management of the underlying causes of ARF (which may include progressive disease, hypercalcemia, or pre-renal azotemia) should be implemented. Adequate hydration is a key component of supportive care for renal dysfunction and failure; however, caution should be exercised to prevent over-hydration and potential cardiac and pulmonary complications.[3] Patients with renal dysfunction should not receive nephrotoxic agents, such as nonsteroidal anti-inflammatory drugs, aminoglycoside antibiotics, and contrast dyes.[3]
To obtain full clinical benefit from treatment, it is important that patients with MM be treated at the maximum effective dose. However, patients with renal dysfunction may require dose reductions because of issues with tolerability. As discussed above, melphalan and lenalidomide should be avoided or used with caution in patients with impaired kidney function, with the dose reduced in patients with renal insufficiency and/or significant cytopenias. Because dexamethasone can lead to fluid retention, patients with impaired renal function who receive the corticosteroid may require diuretics and possibly need dexamethasone dose reductions to manage volume overload. Such dosage modifications can introduce elements of uncertainty into patient treatment and management, potentially leading to under-dosing of patients and subsequent reduced treatment efficacy, or, conversely, can lead to overdosing and increases in toxicity.
In contrast, carfilzomib does not require dose modification in patients with renal impairment, including those receiving dialysis. Dialysis clearance of carfilzomib has not yet been studied.[27] Reduction of effective carfilzomib concentration due to hemodialysis is not expected to be a major concern because carfilzomib is predominantly cleared through extra-renal pathways.[22] Any potential effect of hemodialysis on carfilzomib concentration can be mitigated by administering carfilzomib after hemodialysis[27] so that the administered dose is cleared before the next hemodialysis session begins. Importantly, there were no appreciable differences in the type, frequency, or severity of AEs reported in patients with various degrees of renal function in the PX-171-005 trial compared with those reported in the other phase II carfilzomib trials (in which patients had a CrCl of ≥ 30 mL/min).[22] These findings suggest that pre-existing renal impairment is not likely to be a risk factor for renal and nonrenal AEs following carfilzomib treatment, which stands in contrast with results reported for other agents such as lenalidomide and melphalan[13-16]; however, further studies, particularly large, randomized phase III trials, are needed to confirm these results.
Careful evaluation of blood pressure and appropriate management of anti-hypertensive medications are important measures for reducing the risk of renal AEs in patients receiving treatment. Angiotensin-converting enzyme inhibitors and angiotensin II receptor blockers should be avoided if possible, because these agents may potentiate pre-renal dysfunction.
Small, mostly transient increases in serum creatinine may be observed between two consecutive doses of carfilzomib. These can be managed through oral and intravenous hydration and dose reductions. Although grade 3/4 renal AEs occur infrequently following carfilzomib use, dose reductions or discontinuations may be needed. Using these measures, grade 3/4 renal AEs that are associated with carfilzomib use are often reversible within 1 to 2 weeks. When renal function has recovered to grade 1 or baseline, carfilzomib treatment may be restarted with the dose used before the event or at a reduced dose if the renal AE was determined to be related to carfilzomib.[27] However, it should be noted that there was no association observed between worsening renal function (eg, doubling of serum creatinine level) and the maximum or cumulative dose of carfilzomib received.â©
Conclusion
Renal dysfunction is common in patients with progressive MM and adversely affects their prognosis. For patients with renal impairment, the optimal dose of an anti-myeloma agent that is excreted primarily by the kidneys (eg, lenalidomide) or that is toxic in patients with renal dysfunction (eg, melphalan) may be difficult to determine. Dose adjustments are typically needed, yet this can result in underdosing (which can negatively affect treatment efficacy) or overdosing (which can increase toxicity). The results of the four phase II carfilzomib studies demonstrate that single-agent carfilzomib is safe and well tolerated in patients with baseline renal insufficiency, including those receiving hemodialysis. Despite baseline renal dysfunction in two-thirds of patients who entered the phase II carfilzomib studies, worsening renal function from baseline or grade 3/4 renal AEs were uncommon. Most grouped renal impairment AEs were grade 1/2 and did not result in dose reductions or discontinuations. In a clinical study of patients with various degrees of renal impairment, drug clearance and peak plasma concentration were similar across all groups. Importantly, the carfilzomib dose and schedule do not need to be adjusted in patients with renal impairment. These findings are comparable to what has been previously reported with bortezomib and stand in contrast with the dose modifications needed with lenalidomide and potentially pomalidomide.[17,19,20] Overall, the safety profile reported in the cross-trial analysis supports the conclusion that single-agent carfilzomib is an important therapeutic option for patients with relapsed and/or refractory MM, including those with concomitant renal dysfunction.
Financial Disclosure: Dr. Shah is a consultant to, and receives research support from, Onyx.
Acknowledgment: Medical writing and editorial assistance was provided by BlueMomentum, a division of KnowledgePoint360 Group, San Bruno, California, and funded by Onyx Pharmaceuticals, Inc.
References:
1. Anderson KC, Alsina M, Bensinger W, et al. Multiple myeloma, version 1.2013. J Natl Compr Canc Netw. 2013;11:11-7.
2. Blade J, Rosinol L. Renal, hematologic and infectious complications in multiple myeloma. Best Pract Res Clin Haematol. 2005;18:635-52.
3. Dimopoulos MA, Kastritis E, Rosinol L, et al. Pathogenesis and treatment of renal failure in multiple myeloma. Leukemia. 2008;22:1485-93.
4. Goldschmidt H, Lannert H, Bommer J, Ho AD. Multiple myeloma and renal failure. Nephrol Dial Transplant. 2000;15:301-4.
5. Terpos E, Kastritis E, Roussou M, et al. The combination of bortezomib, melphalan, dexamethasone and intermittent thalidomide is an effective regimen for relapsed/refractory myeloma and is associated with improvement of abnormal bone metabolism and angiogenesis. Leukemia. 2008;22:2247-56.
6. United States Renal Data System. United States Renal Data System 2010 Annual Data Report: Atlas of Chronic Kidney Disease and End-Stage Renal Disease in the United States. Bethesda, Md: National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases; 2010.
7. Faiman BM, Mangan P, Spong J, et al. Renal complications in multiple myeloma and related disorders: survivorship care plan of the International Myeloma Foundation Nurse Leadership Board. Clin J Oncol Nurs. 2011;15(suppl):66-76.
8. Knudsen LM, Hjorth M, Hippe E. Renal failure in multiple myeloma: reversibility and impact on the prognosis. Nordic Myeloma Study Group. Eur J Haematol. 2000;65:175-81.
9. Augustson BM, Begum G, Dunn JA, et al. Early mortality after diagnosis of multiple myeloma: analysis of patients entered onto the United Kingdom Medical Research Council trials between 1980 and 2002-Medical Research Council Adult Leukaemia Working Party. J Clin Oncol. 2005;23:9219-26.
10. Kleber M, Ihorst G, Deschler B, et al. Detection of renal impairment as one specific comorbidity factor in multiple myeloma: multicenter study in 198 consecutive patients. Eur J Haematol. 2009;83:519-27.
11. Blade J, Fernandez-Llama P, Bosch F, et al. Renal failure in multiple myeloma: presenting features and predictors of outcome in 94 patients from a single institution. Arch Intern Med. 1998;158:1889-93.
12. Kyle RA, Gertz MA, Witzig TE, et al. Review of 1027 patients with newly diagnosed multiple myeloma. Mayo Clin Proc. 2003;78:21-33.
13. Carlson K, Hjorth M, Knudsen LM, Nordic Myeloma Study Group. Toxicity in standard melphalan–prednisone therapy among myeloma patients with renal failure–a retrospective analysis and recommendations for dose adjustment. Br J Haematol. 2005; 128:631-35.
14. Dimopoulos MA, Terpos E, Chanan-Khan A, et al. Renal impairment in patients with multiple myeloma: a consensus statement on behalf of the International Myeloma Working Group. J Clin Oncol. 2010;28:4976-84.
15. Dimopoulos M, Alegre A, Stadtmauer EA, et al. The efficacy and safety of lenalidomide plus dexamethasone in relapsed and/or refractory multiple myeloma patients with impaired renal function. Cancer. 2010; 116:3807-14.
16. Niesvizky R, Naib T, Christos PJ, et al. Lenalidomide–induced myelosuppression is associated with renal dysfunction: adverse events evaluation of treatment–naive patients undergoing front–line lenalidomide and dexamethasone therapy. Br J Haematol. 2007; 138:640-3.
17. Revlimid (lenalidomide) capsules prescribing information. Summit, NJ: Celgene Corporation; 2013.
18. Pomalyst (pomalidomide) capsules prescribing information. Summit, NJ: Celgene Corporation; 2013.
19. Chanan-Khan AA, Kaufman JL, Mehta J, et al. Activity and safety of bortezomib in multiple myeloma patients with advanced renal failure: a multicenter retrospective study. Blood. 2007;109:2604-6.
20. Dimopoulos MA, Roussou M, Gkotzamanidou M, et al. The role of novel agents on the reversibility of renal impairment in newly diagnosed symptomatic patients with multiple myeloma. Leukemia. 2013;27: 423-9.
21. Siegel DS, Martin T, Wang M, et al. A phase 2 study of single-agent carfilzomib (PX-171-003-A1) in patients with relapsed and refractory multiple myeloma. Blood. 2012;120:2817-25.
22. Badros AZ, Vij R, Martin T, et al. Carfilzomib in multiple myeloma patients with renal impairment: pharmacokinetics and safety. Leukemia. 2013;27:1707-14.
23. Jagannath S, Vij R, Stewart AK, et al. An open-label single-arm pilot phase ii study (PX-171-003-A0) of low-dose, single-agent carfilzomib in patients with relapsed and refractory multiple myeloma. Clin Lymphoma Myeloma Leuk. 2012;12:310-8.
24. Vij R, Siegel DS, Jagannath S, et al. An open-label, single-arm, phase 2 study of single-agent carfilzomib in patients with relapsed and/or refractory multiple myeloma who have been previously treated with bortezomib. Br J Haematol. 2012;158:739-48.
25. Vij R, Wang M, Kaufman JL, et al. An open-label, single-arm, phase 2 (PX-171-004) study of single-agent carfilzomib in bortezomib-naive patients with relapsed and/or refractory multiple myeloma. Blood. 2012;119:5661-70.
26. Siegel D, Martin T, Nooka A, et al. Integrated safety profile of single-agent carfilzomib: experience from 526 patients enrolled in 4 phase 2 clinical studies. Haematologica. 2013 Aug 9. [Epub ahead of print]
27. Kyprolis (carfilzomib) prescribing information. South San Francisco, Calif: Onyx Pharmaceuticals, Inc.; 2012.
28. Richardson P, Jagannath S, Hussein M, et al. Safety and efficacy of single-agent lenalidomide in patients with relapsed and refractory multiple myeloma. Blood. 2009;114:772-8.
Navigating AE Management for Cellular Therapy Across Hematologic Cancers
A panel of clinical pharmacists discussed strategies for mitigating toxicities across different multiple myeloma, lymphoma, and leukemia populations.