Insights Into the Appropriate Use of New Antimyeloma Therapies
Unfortunately, while survival outcomes with novel therapies have improved, the fraction of patients with multiple myeloma who are cured of their disease remains low. Immune therapies offer the hope for further improvement in outcomes and higher rates of cure.
Oncology (Williston Park). 31(1):64–66.

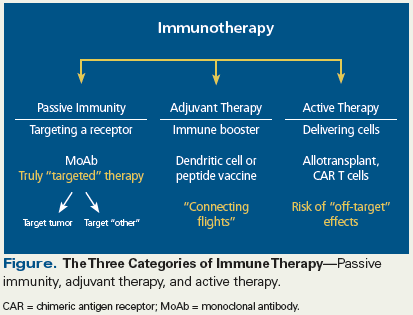
Figure. The Three Categories of Immune Therapy
Over the past decade, survival of patients with multiple myeloma has improved significantly, due in large part to development of new therapeutics that target intracellular signaling in malignant plasma cells. After many years of treatment failure in this disease setting, we are finally seeing clinically meaningful improvements in response duration with the development of myeloma-specific immune therapies. These strategies are well described by Luetkens and colleagues in this issue of ONCOLOGY.[1]
One of the challenges in reviewing this broad topic is to provide the information in a conceptual framework that clinicians can use to guide treatment decisions. To accomplish this task, two paths can be taken; the first involves dividing the large basket of “immune therapy” into three categories: passive immunity, adjuvant therapy, and active therapy (see Figure).[2] The second path is to identify the appropriate clinical setting for use of a given agent.
Passive immunization is currently the most clinically relevant immunotherapy used in the treatment of patients with multiple myeloma. In this approach, monoclonal antibodies directly target specific receptors on myeloma cells. Two antibodies are approved for this purpose by the US Food and Drug Administration: daratumumab, which targets CD38, and elotuzumab, which targets SLAMF7 (signaling lymphocyte activation molecule F7); both agents have recently been reviewed in the medical literature.[3] Monoclonal antibodies not only target the myeloma cell itself, but they can also coordinate the immune response via upregulation of antibody-dependent cellular cytotoxicity and complement-dependent cytotoxicity.[4] Chemoimmunoconjugates that target receptors such as CD38, SLAMF7, or BCMA (B-cell maturation antigen) are also in development. The activity of these agents may not require antibody-dependent cellular cytotoxicity mechanisms, a feature that could be critically important given that multiple myeloma is often associated with underlying immune dysfunction. New monoclonal antibodies targeting the programmed death 1/programmed death ligand 1 axis have also been studied in myeloma, and while ineffective as single agents, in combination with immunomodulatory drugs (lenalidomide and pomalidomide) they have demonstrated significant activity, validating this target in myeloma. Additionally, “off-the-shelf” novel dual targeting antibody strategies are being developed that engage both the myeloma cell and effector T cells. These include bispecific antibody technology targeting BCMA, CD38, or SLAMF7 on the tumor cell, in addition to treatments targeting CD3 on host T cells.
Adjuvant therapy uses a vaccine-based approach to enhance innate immune responses, but clinical outcomes following monotherapy with this type of treatment may not be sufficient to be effective in the short term. Multiple platforms have been evaluated and are under investigation, including peptide-based vaccines and cellular vaccination strategies using dendritic cells. Preliminary results of studies of both approaches confirm that they induce antitumor immune responses, but the question of whether these treatments translate into significant clinical benefit cannot be answered without further study.
Active cellular therapy represents an additional means by which we can manage multiple myeloma by engaging the patient’s own immune system. Allogeneic transplant was the first modality to use active cellular therapy in the treatment of multiple myeloma. It has yielded mixed clinical outcomes in patients with multiple myeloma across a large number of studies, due in large part to high rates of graft-vs-host disease and relapse. As outlined by Luetkens and colleagues, engineered cellular therapy has shown initial promise in multiple myeloma, but the duration of response is often limited. Therapy using chimeric antigen receptor T cells and T-cell receptor–transduced T cells are the two major strategies now under investigation. The issues of identifying the optimal target(s), developing therapies with costimulatory mechanisms of action, and employing strategies that minimize the toxicity of treatment will need to be addressed in carefully designed clinical trials.
As previously noted, the second path of critical importance in providing a conceptual framework for the management of multiple myeloma is to describe the clinical setting in which the specific immune therapy would be most useful. Current treatment for myeloma includes therapies for four different disease states, and the goals of treatment for each of these are different.
In the presymptomatic phase of either monoclonal gammopathy of unknown significance or asymptomatic myeloma, immune strategies could be used to prevent the progression to symptomatic myeloma or the development of a myeloma-defining event. This would be of particular interest if the immune-based treatment was targeting not only the tumor, but also the relationship between tumor cells and the bone marrow microenvironment, since doing so might further disrupt critical molecular survival signals.
In the patient with newly diagnosed symptomatic multiple myeloma, the goal of therapy would be maximal disease control by targeting multiple clones, with the net achievement of minimal residual disease negativity, a particular challenge among high-risk patients.
In the patient with early relapse (failure of one to three prior lines of therapy), the goals are to target the tumor and restore immune function, allowing patients to eventually gain host antitumor immunity and long-term disease control.
Finally, for patients with refractory disease, the sole focus is on symptom burden and disease control, since few other treatment options are available. Based on this framework, different immune strategies will be needed that will likely be partnered with existing agents, and may involve sequencing of immune-based treatments based on where a particular patient is in the trajectory of his or her treatment.
The development of novel therapies has brightened the clinical outlook for patients with multiple myeloma. Unfortunately, while survival outcomes have improved, the fraction of patients with multiple myeloma who are cured of their disease remains low. Immune therapies offer the hope for further improvement in outcomes and higher rates of cure. Disseminating new information about immunotherapies for multiple myeloma in a way that provides a roadmap to approach treatment in the context of disease- and patient-related factors is essential to improving our patients’ quality of life and clinical outcomes.
Financial Disclosure:Dr. Kaufman is a consultant to Celgene, Seattle Genetics, and Sutro Pharmaceuticals; serves on the data safety monitoring boards of Incyte, Pharmacyclics, and TG Therapeutics; and receives research funding from Merck, Novartis, and Onyx. Dr. Lonial serves as a consultant to Bristol Myers-Squibb, Celgene, Janssen, Merck, Novartis, Onyx, and Takeda.
References:
1. Luetkens T, Yousef S, Radhakrishnan SV, Atanackovic D. Current strategies for the immunotherapy of multiple myeloma. Oncology (Williston Park). 2017;31:55-63.
2. Neri P, Bahlis NJ, Lonial S. New strategies in multiple myeloma: immunotherapy as a novel approach to treat patients with multiple myeloma. Clin Cancer Res. 2016 Oct 19. [Epub ahead of print]
3. Hofmeister C, Lonial S. How to integrate elotuzumab and daratumumab into therapy for multiple myeloma. J Clin Oncol. 2016 Oct 31. [Epub ahead of print]
4. Tai YT, Anderson KC. Antibody-based therapies in multiple myeloma. Bone Marrow Res. 2011;2011:924058.
Navigating AE Management for Cellular Therapy Across Hematologic Cancers
A panel of clinical pharmacists discussed strategies for mitigating toxicities across different multiple myeloma, lymphoma, and leukemia populations.
