Integrating Novel Targets and Precision Medicine Into Prostate Cancer Care-Part 1: The Non-Androgen–Targetable Pathways in Castration-Resistant Prostate Cancer
Here, we review the current use and future direction of novel therapeutic targets in the treatment of castration-resistant prostate cancer.
Oncology (Williston Park). 33(3):113-8.

Bryden Considine, DO

Daniel P. Petrylak, MD

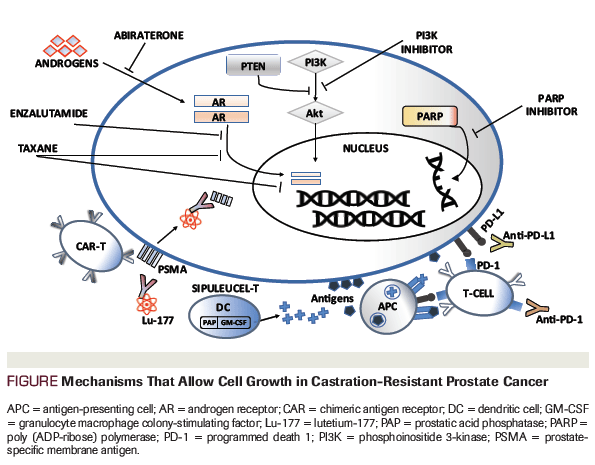
Figure. Mechanisms That Allow Cell Growth in Castration-Resistant Prostate Cancer
Prostate cancer is the second most common cause of cancer-related death in men. Although the overall survival of patients with metastatic prostate cancer has improved with the addition of second-generation hormone therapy, most men will develop progressive disease, eventually leading to death. Novel therapeutic mechanisms are needed to improve treatments and outcomes in patients with metastatic prostate cancer. Biomarker-driven treatments such as targeted therapies and immuno-oncologic agents are currently under investigation, and may lead to less toxicity and better outcomes for these patients. Here, we review the current use and future direction of these novel therapeutic targets.
Introduction
Prostate cancer is the most common cancer in men in the United States, ranking second to lung cancer among deaths from cancer, with an estimated 29,430 deaths in 2018.[1] The most lethal form of the disease is castration-resistant prostate cancer (CRPC), which occurs with the loss of clinical response to initial androgen deprivation therapy (ADT). The treatment for CRPC has evolved over time, from treatments with a palliative focus to treatments that extend survival. Docetaxel was the first agent found to improve survival in men with CRPC, with a median survival of 19 months.[2,3] There have been five new drugs approved by the US Food and Drug Administration (FDA) for prostate cancer since 2010, which extend survival but are not curative. These new agents include the second-generation hormonal agents abiraterone and enzalutamide, which target the androgen receptor (AR) pathway, as well as non-androgen–targeted therapies, including chemotherapy such as cabazitaxel, DNA-damaging agents such as radium-223, and immunotherapy such as sipuleucel-T.[4] Despite these advances, each class of drugs improves the median survival by only approximately 3 to 4 months, thus stressing the need for novel therapeutic approaches.
Despite the use of ADT in CRPC, cell growth and proliferation continues. The mechanisms that allow cell growth in this clinical state include, but are not limited to, autocrine androgen signaling, AR overexpression, and AR splice variants.[5] Further understanding of these mechanisms has led to the development of the FDA-approved agents (Figure). The aim of this review is to examine the FDA-approved non-androgen–mediatedtherapeutic targets, as well as promising agents in clinical trials for patients with CRPC.
Treatment Options for Metastatic CRPC Patients
Taxane chemotherapy
The synthetic taxane docetaxel was the first agent found to show a survival benefit in patients with metastatic CRPC, as demonstrated in two phase III trials, the Southwest Oncology Group 99-16 trial and the TAX 327 trial.[2,3] These two studies established the benefit of taxane chemotherapy and set a new standard of care in the treatment of metastatic CRPC. Despite the survival benefit demonstrated with docetaxel, all patients ultimately progress on treatment due to either innate or acquired taxane resistance.
Cabazitaxel was developed due to its low affinity for MDR1 and superior blood-brain barrier penetration compared with paclitaxel and docetaxel.[6,7] Its efficacy was investigated in the second-line setting in the TROPIC trial, which included men with metastatic CRPC who had progressed during or after docetaxel treatment. This phase III study found an increased overall survival of 15.1 months in patients treated with cabazitaxel, compared with 12.7 months in men treated with mitoxantrone.[8] This was the first agent to demonstrate a survival benefit in docetaxel-refractory patients, and this trial led to the FDA approval of cabazitaxel in 2010.
The FIRSTANA trial investigated the use of cabazitaxel vs docetaxel in the first-line setting for metastatic CRPC. Patients were randomized 1:1:1 to cabazitaxel 20 mg/m2 (C20), cabazitaxel 25 mg/m2 (C25), or docetaxel 75 mg/m2 (D75). The median overall survival was 24.5 months with C20, 25.2 months with C25, and 24.3 months with D75. Adverse events ≥ grade 3 occurred in 41.2% of patients treated with C20, 60.1% of those treated with C25, and 46.0% with D75. Febrile neutropenia was most frequently seen in patients who received C25. Overall, this study showed that cabazitaxel is not superior to docetaxel in the first-line treatment of metastatic CRPC.[9] Despite this finding, there are instances when cabazitaxel could be considered instead of docetaxel. Specifically, cabazitaxel was found to have lower rates of grade 3/4 peripheral neuropathy and may be a better option for certain patients.
Immunologic targets
Advances in cancer immunology have changed the treatment and prognosis for many advanced malignancies. These immunotherapeutic agents include cancer vaccines, immune checkpoint inhibitors, adoptive T-cell therapy, and viral vectors. Sipuleucel-T, an autologous dendritic cell vaccine, was approved by the FDA in 2010 for prostate cancer. After leukapheresis, harvested antigen-presenting cells are exposed to a fusion protein of prostatic acid phosphatase (PAP) and granulocyte-macrophage colony-stimulating factor (GM-CSF) (referred to as PA2024) and infused back into the patient.[10] This process is repeated every 2 weeks for 3 cycles. The phase III IMPACT trial randomized men with metastatic CRPC 2:1 to receive sipuleucel-T or placebo and found no significant difference in time to progression, but found an improved median overall survival in the sipuleucel-T group compared with the placebo group (25.8 months vs 21.7 months).[11] Survival appears to be longer in patients with low-volume disease. The exact mechanism by which these patients experienced improvement in overall survival without a difference in time to progression remains unclear. It is possible that the effect that these immune agents provide is delayed beyond initial progression, in comparison with traditional chemotherapy in which a decrease in time to progression is more closely related to overall survival. This study led to the FDA approval of sipuleucel-T for the treatment of asymptomatic or minimally symptomatic patients with nonvisceral metastatic CRPC in 2010.
The use of cancer vaccines, in an effort to stimulate a tumor-directed immune response, is an area of ongoing clinical research and has led to the development of a prostate-specific antigen (PSA)-based vaccine called PSA-TRICOM. This vaccine consists of the vaccinia and fowlpox viral vectors and contains the PSA gene and the costimulatory molecules B7.1, ICAM-1, and LFA-3, referred to as TRICOM. The proposed mechanism of this vaccine is thought to be through the presentation of PSA to immune cells with costimulatory molecules, which enhances a T-cell cytotoxic response.[12] This was first investigated in a phase II trial of men with minimally symptomatic metastatic CRPC randomized 2:1 to PSA-TRICOM and GM-CSF or placebo with an empty vector and GM-CSF. Although this study did not show any difference in progression-free survival, it did show an increase of 25.1 months in median overall survival with PSA-TRICOM compared with 16.6 months with placebo.[13] These findings led to a phase III study; unfortunately, no survival benefit was demonstrated with PSA-TRICOM.[14,15]
Chimeric antigen receptor (CAR) T cells are autologous T cells that are engineered ex vivo to express a recombinant AR.[16] The goal of CAR T-cell therapy is to direct immune activation against the target antigen. CAR T-cell therapy has had recent success in the treatment of hematologic malignancies, with FDA approval for the treatment of refractory diffuse large B-cell lymphoma and refractory B-cell acute lymphoblastic leukemia. Prostate cancer is a viable candidate for CAR T-cell development, since it has specific tumor-associated antigens, including prostate-specific membrane antigen (PSMA) and prostate stem cell antigen (PSCA).[17] A phase I study using PSMA-directed CAR T cells reported that 2 of 5 patients treated obtained a PSA response.[18] A second phase I study using a PSMA-directed CAR T cell is ongoing (ClinicalTrials.gov identifier: NCT01140373). One of the challenges identified in treating metastatic prostate cancer with CAR T-cell therapy is related to the high prevalence of bony disease and the difficulty of recruiting CAR T cells to these lesions, overcoming the immunosuppressive environment.[19]
The success of checkpoint inhibitors that target either cytotoxic T-lymphocyte–associated antigen 4 (CTLA-4) or the programmed death 1 (PD-1)/programmed death ligand 1 (PD-L1) axis has changed the way we treat many advanced malignancies, and they continue to gain additional indications from the FDA. The potential promise of immunotherapy in the treatment of prostate cancer is based on findings that show a significant T-cell infiltration in prostate tissue.[20] Phase I/II studies of ipilimumab in metastatic CRPC showed efficacy, leading to the phase III CA184-043 trial, which included patients with bony metastases who had progressed on docetaxel therapy. In this study, patients received 8 Gy of radiation to at least one bony site, followed by either ipilimumab 10 mg/kg or placebo every 3 weeks for a maximum of 4 cycles, with the option of maintenance therapy every 3 months in non-progressing patients tolerating treatment. The median overall survival did not meet statistical significance between the ipilimumab and placebo groups (11.2 vs 10.0 months).[21]
However, a subgroup analysis observed an improved overall survival with ipilimumab in patients with more favorable prognostic features, including those without visceral metastasis, those with no increase or a minimal increase in alkaline phosphatase levels, and those without anemia.[21] The phase III CA184-095 trial investigated the safety and efficacy of ipilimumab vs placebo in chemotherapy-naive patients, and found no significant difference in survival between the groups.[22]
PD-1 has been shown to be a target in prostate cancer, since tumor-infiltrating lymphocytes found in prostate cancer have been shown to express PD-1 and PD-L1, albeit at a lower rate than other solid tumors.[23,24] However, in an early study with the anti–PD-1 agent nivolumab, though tumor responses were demonstrated among patients with solid tumors, there were no responses seen in the 17 patients with CRPC.[25] Tumor samples from only two of these patients were tested for PD-L1 expression and were negative. None of these patients received prior treatment with either abiraterone or enzalutamide, which may change the tumor microenvironment. The combination of ipilimumab and nivolumab is currently being investigated in the phase II CheckMate 650 trial (ClinicalTrials.gov identifier: NCT02985957).
Despite this lack of response to immune checkpoint inhibition, Graff et al identified two exceptional responders, raising the hypothesis that AR blockade may enhance response to immunotherapy, perhaps through upregulation of PD-L1.[26,27] A phase II trial evaluated patients who had progressed on enzalutamide; they were given pembrolizumab every 3 weeks for 4 doses in addition to enzalutamide. Three of the first 10 patients enrolled had a remarkable decrease in PSA levels; of the 2 patients with measurable disease, both had a partial response.[28] This unexpected finding may be explained by a study that demonstrated that patients who had progressed on enzalutamide have a significantly increased number of PD-1/2 dendritic cells in their blood compared with treatment-naive patients responding to enzalutamide.[29]. Pembrolizumab may also have activity in prostate cancer patients with microsatellite instability, for which it is FDA approved. It should be noted that only about 2% of CRPC patients are microsatellite instability–high.
Other checkpoint inhibitors are currently being evaluated in CRPC. A phase I study of atezolizumab in patients with metastatic CRPC who had progressed on prior therapy with either enzalutamide and/or sipuleucel-T was recently performed. Of 15 patients, 1 patient had a partial response, 5 patients had stable disease, and 2 experienced a ≥ 50% decrease in PSA and a 12-month overall survival rate of 55.6%. Only 9 of the patients experienced ≤ grade 3 adverse events, and there were no grade 4 or 5 events, demonstrating that atezolizumab was well tolerated and provided long-term disease control in this heavily pretreated population.[30] Currently, a phase III randomized multicenter trial is investigating the combination of atezolizumab with enzalutamide compared with enzalutamide alone in patients who have received prior abiraterone therapy and have either progressed on, are ineligible for, or refused taxane therapy (ClinicalTrials.gov identifier: NCT03016312).
Membrane and Cellular Targets
PSMA
PSMA is a transmembrane protein that is highly specific for both benign and malignant prostate epithelial cells.[31,32] PSMA is also expressed on vascular endothelium, as well as other epithelial tissues, including salivary glands. PSMA is an ideal therapeutic target, since it is membrane bound, and ligand binding leads to internalization of the molecule.[33] This has led to the development of multiple PSMA-directed therapies, including antibody-drug conjugates. This directed therapy aims to deliver the drug molecules to PSMA expression cells, sparing normal tissues and potential associated side effects. MLN2704 is an antibody-drug conjugate comprised of HuJ591, a humanized anti-PSMA monoclonal antibody, and the microtubule inhibitor DM1. In early-phase studies, a low rate of clinical activity was seen, which was attributed to poor drug delivery secondary to deconjugation of MLN2704.[34] A phase II trial with monomethyl auristatin E, a synthetic antimitotic agent, conjugated with a PSMA monoclonal antibody demonstrated a PSA response > 30% in 30% of patients. Treatment toxicities were common, with 25% of previously taxane-exposed patients developing ≥ grade 3 neutropenia.[35,36]
BIND-014 is a docetaxel-containing PSMA-targeted nanoparticle. These nanoparticles are designed to accumulate in prostate tissue and release docetaxel in a controlled manner.[32,37,38] A phase II study was completed that included 42 docetaxel-naive patients with metastatic CRPC who had previously been treated with abiraterone, enzalutamide, or both. The median radiographic progression–free survival was 7.1 months, with a PSA decline of ≥ 50% seen in 30% of patients. Unfortunately, rates of toxicity were high; the most frequently seen adverse events were fatigue (69%), nausea (55%), and diarrhea (45%), followed by neuropathy, lymphopenia, and anemia. Overall, the toxicity profile and clinical activity of BIND-014 was similar to systemic docetaxel administration, which raised concerns for drug deconjugation and target affinity.[37,38]
Conjugates with radioisotopes allow for targeted delivery of alpha and beta particles to the PSMA-expressing site. Lutetium-177 (177Lu)-PSMA-617 complexes have shown preliminary activity. In retrospective studies, the percentage of patients who experienced a > 50% reduction in PSA levels has been reported to be 30% to 70%. Common side effects included hematologic and salivary gland toxicities.[39] In a phase II trial of 47 heavily pretreated men with metastatic CRPC, patients were given 177Lu conjugated with HuJ591; 36.2% had a > 30% PSA response and a median overall survival of 17.6 months. All patients experienced transient hematologic toxicity, with 46.8% of patients having grade 4 thrombocytopenia.[40] These responses seen in heavily pretreated patients are exciting and have provided the foundation for ongoing prospective clinical trials.
Phosphoinositide 3-kinase (PI3K)
The PI3K signaling pathway regulates cellular processes essential for cell proliferation and growth. The PI3K signaling cascade leads to AKT activation by phosphorylation of pyruvate dehydrogenase kinase 1 (PDK1) and mammalian target of rapamycin (mTOR). Both the AR and PI3K pathways are implicated in prostate cancer, with the PI3K pathway shown to have a role in the development of prostate cancer. Activation of the PI3K signaling cascade is associated with advanced disease and poor outcomes in patients with prostate cancer.[41-47] Carver et al and Mulholland et al independently demonstrated the relationship between the AR and PI3K signaling pathways. With PTEN loss, the PI3K/AKT pathway is activated, leading to prostate cancer cell growth independent of AR signaling. With potent second-generation AR blockade, there may be selection for tumor cells with PI3K activation that are able to grow independent of the AR in a castration-resistant manner.[41,48] Loss of PTEN, which has been identified in 60% of CRPCs, leads to continued activation of PI3K and has been identified as a factor leading to prostate cancer development.[49]
Due to the implications of the PI3K pathway in cancer development and pathogenesis, it has been a popular pharmacologic target for drug development. Multiple classes of drugs have been developed targeting PI3K, as well as downstream targets such as mTOR and AKT.[46] Despite these efforts, early studies of PI3K inhibitors have shown limited efficacy in prostate cancer. A phase II study looking at PX-866, a pan-isoform inhibitor of PI3K, included 43 docetaxel-naive patients with metastatic CRPC. Eleven patients were progression-free at 12 weeks, with only 1 patient having a confirmed PSA response.[50] Burris et al investigated the pan-AKT inhibitor GSK2141795 in 9 patients with prostate cancer, 2 of whom had a partial or complete loss of PTEN and stable disease for over 6 months.[51]
Rathkopf et al investigated the concurrent blockade of mTOR with everolimus and epidermal growth factor receptor inhibition with gefitinib in patients with CRPC.[52] The combination of gefitinib and everolimus did not result in antitumor activity; however, it was found that 13 of 37 patients experienced a rapid increase in PSA, which decreased upon stopping this medication. This finding suggests that the PI3K signaling pathway inhibits the AR and that blockade of the PI3K signaling pathway promotes AR activity. The increase in PSA observed may be a marker of AR reactivation in these patients.[46] This study led to investigations testing concurrent PI3K inhibitors and androgen blockade.[52]
In part B of the phase II study of the PI3K inhibitor PX-866 by Hotte et al, the addition of androgen blockade with abiraterone was investigated in an attempt to reverse acquired androgen resistance. Twenty-five patients with metastatic CRPC who had progressed on abiraterone/prednisone were included in the study and received PX-866. Unfortunately, the addition of PX-866 to abiraterone and prednisone did not result in any antitumor effect. This raised the question of whether this combination should be used earlier in the disease course, prior to the development of castration resistance.[53]
A phase II study of concurrent AKT inhibition with AR blockade with ipatasertib and abiraterone found evidence for both improved progression-free survival and overall survival in patients with metastatic CRPC. In this study, 253 patients with metastatic CRPC who had previously been treated with docetaxel were included and randomized 1:1:1 to either ipatasertib 400 mg, ipatasertib 200 mg, or placebo, all in combination with abiraterone/prednisone. When comparing ipatasertib 400 mg vs placebo, the progression-free survival was 8.2 vs 6.4 months, and overall survival was 18.9 vs 15.6 months. In addition, patients with loss of PTEN were found to have superior progression-free survival when treated with ipatasertib 400 mg vs placebo.[54] Within this study, only 23 of the 253 patients had received novel anti-androgen therapy prior to investigation. It is possible that this combination of AKT and AR inhibition may have clinical benefit if used earlier in the disease course, prior to the development of castration resistance; this is the focus of an ongoing phase III clinical trial.[55]
Conclusion
There have been significant advances in the treatment of CRPC; however, options remain limited, and complete eradication of disease has yet to be achieved. Clinical trials have evolved to incorporate molecular targets, such as PSMA, PI3K, and PD-L1. Identification of patients who are likely to respond to these targeted therapies based upon their molecular profiles has the potential to improve efficacy, prolong survival, and reduce unnecessary exposure to drug toxicities.
Financial Disclosure:Dr. Petrylak receives consultant fees and grant support from Ada Cap, Astellas, AstraZeneca, Bayer, Bristol-Myers Squibb, Clovis, Eli Lilly, Pfizer, Roche Laboratories, and Seattle Genetics; he also receives consultant fees from Amgen, Boehringer Ingelheim, Exelixis, Incyte, Janssen, Pharmacyclics, and Urogen, and grant support from Endocyte, Genentech, Innocrin, MedImmune, Merck, Novartis, Progenics, and Sanofi Aventis. He also has ownership interest in Bellicum and Tyme.
PERSPECTIVE
The Evolution of Immunotherapy in Metastatic CRPC Is Exciting, but Caution is Paramount
Sonia M. Seng, MD
One in 8 men will develop prostate cancer in his lifetime, and most will progress to metastatic castration-resistant prostate cancer (mCRPC), the most aggressive form of this disease. In this review, Considine and Petrylak summarize effective systemic therapies for mCRPC associated with improved survival and highlight promising investigational approaches to treatment.
As a community oncologist who treats multiple types of malignancies, it is exciting to see the evolution of immunotherapy trials in prostate cancer. Checkpoint inhibitors have the ability to produce deep and durable responses and to extend survival in the frontline setting; they can also be effective in treatment-refractory patients. What has eluded us, however, is the ability to precisely identify predictive biomarkers for responders.
We know that programmed death 1 (PD-1)/programmed death ligand 1 (PD-L1) expression, microsatellite instability (MSI) status or its correlate mismatch repair protein expression, and tumor mutational burden (TMB) can predict for response to checkpoint inhibitors. However, more practical questions arise as we accrue more experience with these agents and biomarkers. In lung cancer, we have learned that patients with high PD-L1 expression are unlikely to respond to checkpoint inhibitors in the presence of other oncogenic drivers, such as EGFR, ALK, and ROS1. However, TMB has been reported to be an independent predictor of response despite the presence of other oncogenic drivers.
Earlier trials of the cytotoxic T-lymphocyte–associated antigen 4 inhibitor ipilimumab and the PD-1 inhibitor nivolumab were disappointing. The authors highlight the phase III CA184-095 trial, which illustrated no survival advantage for ipilimumab vs placebo in chemotherapy-naive patients. No improvement in outcomes was seen in the phase III CA184-043 trial, either, which evaluated radiation plus ipilimumab vs placebo in the post-docetaxel setting. Disappointingly, none of the 17 CRPC patients responded to treatment in an early nivolumab trial.
Drawing from other malignancies, the outcomes may be more promising with combination immunotherapy, and by enriching for a population that is more likely to respond. In KEYNOTE-199, de Bono reported on the activity of pembrolizumab in patients with docetaxel-refractory mCRPC and noted increased response rates in patients with somatic BRCA1/2 or ATM mutations. A multitude of trials evaluating combination therapy and enriching for special mCRPC populations are underway. Pembrolizumab is being studied in combination with radium-223 (ClinicalTrials.gov identifier: NCT03093428). KEYNOTE-365 is a four-arm trial evaluating the combination of pembrolizumab with either olaparib, docetaxel and prednisone, enzalutamide, or abiraterone and prednisone (NCT02861573). Nivolumab is currently being evaluated in patients with mCRPC with DNA repair defects (NCT03040791) or CDK12 mutation (NCT03570619), as well as in combination with rucaparib, docetaxel, or enzalutamide (NCT03338790). As reported, a phase III trial of atezolizumab and enzalutamide vs enzalutamide is also underway (NCT03016312).
As responsible oncologists, we must be careful not to oversell the promise of immunotherapy to the majority of patients, who are unlikely to benefit, or to gloss over the many serious side effects associated with these treatments. It will also be important to incorporate clinical value and cost into immunotherapy trials given the number of patients that will ultimately be eligible for this type of therapy. The authors report on other promising agents in mCRPC, including prostate-specific membrane antigen conjugates, including lutetium-177, as well as phosphoinositide 3-kinase inhibitors. Until more is known about the efficacy of these investigational approaches, I encourage my patients with mCRPC to undergo genetic testing, consider clinical trials, and undergo comprehensive genomic profiling to evaluate for a personalized approach.
Financial Disclosure:Dr. Seng has no significant financial interest in or other relationship with the manufacturer of any product or provider of any service mentioned in this article.
Dr. Seng is the Director of Oncology Clinical Trials at Southcoast Centers for Cancer Care, Fairhaven and Fall River, Massachusetts.
References:
1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin. 2018;68:7-30.
2. Petrylak DP, Tangen CM, Hussain MH, et al. Docetaxel and estramustine compared with mitoxantrone and prednisone for advanced refractory prostate cancer. N Engl J Med. 2004;351:1513-20.
3. Tannock IF, de Wit R, Berry WR, et al. Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer. N Engl J Med. 2004;351:1502-12.
4. Suzman DL, Antonarakis ES. Castration-resistant prostate cancer: latest evidence and therapeutic implications. Ther Adv Med Oncol. 2014;6:167-79.
5. Reichert ZR, Hussain M. Androgen receptor and beyond, targeting androgen signaling in castration-resistant prostate cancer. Cancer J. 2016;22:326-9.
6. Mita AC, Denis LJ, Rowinsky EK, et al. Phase I and pharmacokinetic study of XRP6258 (RPR 116258A), a novel taxane, administered as a 1-hour infusion every 3 weeks in patients with advanced solid tumors. Clin Cancer Res. 2009;15:723-30.
7. Palmieri D, Chambers AF, Felding-Habermann B, et al. The biology of metastasis to a sanctuary site. Clin Cancer Res. 2007;13:1656-62.
8. de Bono JS, Oudard S, Ozguroglu M, et al. Prednisone plus cabazitaxel or mitoxantrone for metastatic castration-resistant prostate cancer progressing after docetaxel treatment: a randomised open-label trial. Lancet. 2010;376:1147-54.
9. Oudard S, Fizazi K, Sengeløv L, et al. Cabazitaxel versus docetaxel as first-line therapy for patients with metastatic castration-resistant prostate cancer: a randomized phase III trial-FIRSTANA. J Clin Oncol. 2017;35:3189-97.
10. So-Rosillo R, Small EJ. Sipuleucel-T (APC8015) for prostate cancer. Expert Rev Anticancer Ther. 2006;6:1163-7.
11. Kantoff PW, Higano CS, Shore ND, et al. Sipuleucel-T immunotherapy for castration-resistant prostate cancer. N Engl J Med. 2010;363:411-22.
12. Madan RA, Arlen PM, Mohebtash M, et al. Prostvac-VF: a vector-based vaccine targeting PSA in prostate cancer. Expert Opin Investig Drugs. 2009;18:1001-11.
13. Kantoff PW, Schuetz TJ, Blumenstein BA, et al. Overall survival analysis of a phase II randomized controlled trial of a Poxviral-based PSA-targeted immunotherapy in metastatic castration-resistant prostate cancer. J Clin Oncol. 2010;28:1099-105.
14. Gulley JL, Giacchino JL, Breitmeyer JB, et al. Prospect: a randomized double-blind phase 3 efficacy study of PROSTVAC-VF immunotherapy in men with asymptomatic/minimally symptomatic metastatic castration-resistant prostate cancer. J Clin Oncol. 2017;33(15 suppl):abstr TPS5081.
15. Gulley JL, Borre M, Vogelzang NJ, et al. Results of PROSPECT: a randomized phase 3 trial of PROSTVAC-V/F (PRO) in men with asymptomatic or minimally symptomatic metastatic, castration-resistant prostate cancer. J Clin Oncol. 2018;36(15 suppl):abstr 5006.
16. Sadelain M, Brentjens R, Riviere I. The basic principles of chimeric antigen receptor design. Cancer Discov. 2013;3:388-98.
17. Hillerdal V, Ramachandran M, Leja J, Essand M. Systemic treatment with CAR-engineered T cells against PSCA delays subcutaneous tumor growth and prolongs survival of mice. BMC Cancer. 2014;14:30.
18. Junghans RP, Ma Q, Rathore R, et al. Phase I trial of anti-PSMA designer CAR-T cells in prostate cancer: possible role for interacting interleukin 2-T cell pharmacodynamics as a determinant of clinical response. Prostate. 2016;76:1257-70.
19. Hillerdal V, Essand M. Chimeric antigen receptor-engineered T cells for the treatment of metastatic prostate cancer. BioDrugs. 2015;29:75-89.
20. Bronte V, Kasic T, Gri G, et al. Boosting antitumor responses of T lymphocytes infiltrating human prostate cancers. J Exp Med. 2005;201:1257-68.
21. Kwon ED, Drake CG, Scher HI, et al. Ipilimumab versus placebo after radiotherapy in patients with metastatic castration-resistant prostate cancer that had progressed after docetaxel chemotherapy (CA184-043): a multicentre, randomised, double-blind, phase 3 trial. Lancet Oncol. 2014;15:700-12.
22. Beer TM, Kwon ED, Drake CG, et al. Randomized, double-blind, phase III trial of ipilimumab versus placebo in asymptomatic or minimally symptomatic patients with metastatic chemotherapy-naive castration-resistant prostate cancer. J Clin Oncol. 2017;35:40-7.
23. Sfanos KS, Bruno TC, Maris CH, et al. Phenotypic analysis of prostate-infiltrating lymphocytes reveals TH17 and Treg skewing. Clin Cancer Res. 2008;14:3254-61.
24. Ebelt K, Babaryka G, Frankenberger B, et al. Prostate cancer lesions are surrounded by FOXP3+, PD-1+ and B7-H1+ lymphocyte clusters. Eur J Cancer. 2009;45:1664-72.
25. Topalian SL, Hodi FS, Brahmer JR, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med. 2012;366:2443-54.
26. Graff JN, Drake CG, Beer TM. Complete biochemical (prostate-specific antigen) response to sipuleucel-T with enzalutamide in castration-resistant prostate cancer: a case report with implications for future research. Urology. 2013;81:381-3.
27. Graff JN, Puri S, Bifulco CB, et al. Sustained complete response to CTLA-4 blockade in a patient with metastatic, castration-resistant prostate cancer. Cancer Immunol Res. 2014;2:399-403.
28. Graff JN, Alumkal JJ, Drake CG, et al. Early evidence of anti-PD-1 activity in enzalutamide-resistant prostate cancer. Oncotarget. 2016;7:52810-7.
29. Bishop JL, Sio A, Angeles A, et al. PD-L1 is highly expressed in enzalutamide resistant prostate cancer. Oncotarget. 2015;6:234-42.
30. Kim JW, Shaffer DR, Massard C, et al. A phase Ia study of safety and clinical activity of atezolizumab (atezo) in patients (pts) with metastatic castration-resistant prostate cancer (mCRPC). J Clin Oncol. 2018;36(suppl):abstr 187.
31. Tsourlakis MC, Klein F, Kluth M, et al. PSMA expression is highly homogenous in primary prostate cancer. Appl Immunohistochem Mol Morphol. 2015;23:449-55.
32. Teo MY, Morris MJ. Prostate-specific membrane antigen–directed therapy for metastatic castration-resistant prostate cancer. Cancer J. 2016;22:347-52.
33. Liu H, Rajasekaran AK, Moy P, et al. Constitutive and antibody-induced internalization of prostate-specific membrane antigen. Cancer Res. 1998;58:4055-60.
34. Galsky MD, Eisenberger M, Moore-Cooper S, et al. Phase I trial of the prostate-specific membrane antigen-directed immunoconjugate MLN2704 in patients with progressive metastatic castration-resistant prostate cancer. J Clin Oncol. 2008;26:2147-54.
35. Petrylak DP, Smith DC, Appleman LJ, et al. A phase II trial of prostate-specific membrane antigen antibody drug conjugate (PSMA ADC) in taxane-refractory metastatic castration-resistant prostate cancer (mCRPC). J Clin Oncol. 2014;32(suppl):abstr 83.
36. Petrylak DP, Vogelzang NJ, Chatta GS, et al. A phase 2 study of prostate specific membrane antigen antibody drug conjugate (PSMA ADC) in patients (pts) with progressive metastatic castration-resistant prostate cancer (mCRPC) following abiraterone and/or enzalutamide (abi/enz). J Clin Oncol. 2015;33(suppl):abstr 144.
37. Von Hoff DD, Mita MM, Ramanathan RK, et al. Phase I study of PSMA-targeted docetaxel-containing nanoparticle BIND-014 in patients with advanced solid tumors. Clin Cancer Res. 2016;22:3157-63.
38. Autio KA, Garcia JA, Alva AS, et al. A phase 2 study of BIND-014 (PSMA-targeted docetaxel nanoparticle) administered to patients with chemotherapy-naïve metastatic castration-resistant prostate cancer (mCRPC). J Clin Oncol. 2016;34(suppl):abstr 233.
39. Emmett L, Willowson K, Violet J, et al. Lutetium (177) PSMA radionuclide therapy for men with prostate cancer: a review of the current literature and discussion of practical aspects of therapy. J Med Radiat Sci. 2017;64:52-60.
40. Tagawa ST, Milowsky MI, Morris M, et al. Phase II study of Lutetium-177-labeled anti-prostate-specific membrane antigen monoclonal antibody J591 for metastatic castration-resistant prostate cancer. Clin Cancer Res. 2013;19:5182-91.
41. Carver BS, Chapinski C, Wongvipat J, et al. Reciprocal feedback regulation of PI3K and androgen receptor signaling in PTEN-deficient prostate cancer. Cancer Cell. 2011;19:575-86.
42. Bitting RL, Armstrong AJ. Targeting the PI3K/Akt/mTOR pathway in castration-resistant prostate cancer. Endocr Relat Cancer. 2013;20:R83-R99.
43. Jiao J, Wang S, Qiao R, et al. Murine cell lines derived from Pten null prostate cancer show the critical role of PTEN in hormone refractory prostate cancer development. Cancer Res. 2007;67:6083-91.
44. Liu L, Dong X. Complex impacts of PI3K/AKT inhibitors to androgen receptor gene expression in prostate cancer cells. PLoS One. 2014;9:e108780.
45. Reid AH, Attard G, Ambroisine L, et al. Molecular characterisation of ERG, ETV1 and PTEN gene loci identifies patients at low and high risk of death from prostate cancer. Br J Cancer. 2010;102:678-84.
46. Crumbaker M, Khoja L, Joshua AM. AR signaling and the PI3K pathway in prostate cancer. Cancers (Basel). 2017;9:E34.
47. Taylor BS, Schultz N, Hieronymus H, et al. Integrative genomic profiling of human prostate cancer. Cancer Cell. 2010;18:11-22.
48. Mulholland DJ, Tran LM, Li Y, et al. Cell autonomous role of PTEN in regulating castration-resistant prostate cancer growth. Cancer Cell. 2011;19:792-804.
49. Vivanco I, Sawyers CL. The phosphatidylinositol 3-kinase AKT pathway in human cancer. Nat Rev Cancer. 2002;2:489-501.
50. Hotte SJ, Eisenhauer EA, Joshua AM, et al. NCIC CTG, IND-205: a phase II study of PX-866 in patients with recurrent or metastatic castration-resistant prostate cancer (CRPC). J Clin Oncol. 2013;31(suppl):abstr 5042.
51. Burris HA, Siu LL, Infante JR, et al. Safety, pharmacokinetics (PK), pharmacodynamics (PD), and clinical activity of the oral AKT inhibitor GSK2141795 (GSK795) in a phase I first-in-human study. J Clin Oncol. 2011;29(suppl):abstr 3003.
52. Rathkopf DE, Larson SM, Anand A, et al. Everolimus combined with gefitinib in patients with metastatic castration-resistant prostate cancer: phase 1/2 results and signaling pathway implications. Cancer. 2015;121:3853-61.
53. Hotte SJ, Joshua AM, Torri V, et al. IND 205B: a phase II study of the PI3K inhibitor PX-866 and continued abiraterone/prednisone in patients with recurrent or metastatic castration resistant prostate cancer (CRPC) with PSA progression on abiraterone/prednisone. J Clin Oncol. 2015;33(suppl):abstr 279.
54. de Bono JS, de Giorgi U, Massard C, et al. Randomized phase II study of AKT blockade with ipatasertib (GDC-0068) and abiraterone (Abi) vs. Abi alone in patients with metastatic castration-resistant prostate cancer (mCRPC) after docetaxel chemotherapy (A. MARTIN Study). J Clin Oncol. 2016;34(suppl):abstr 5017.
55. de Bono J, Bracarda S, Chi K, et al. Randomized phase III trial of ipatasertib vs. placebo, plus abiraterone and prednisone/prednisolone, in men with asymptomatic or mildly symptomatic previously untreated metastatic castrate-resistant prostate cancer (mCRPC). Ann Oncol. 2017;28(suppl 5):abstr 834TiP.
