Efficacy of PARP Inhibitors as Maintenance Therapy for Metastatic Castration-Resistant Prostate Cancer: A Meta-Analysis of Randomized Controlled Trials
The aim of this meta-analysis is to analyze the efficacy of these drugs in the treatment of mCRPC in terms of progression-free survival (PFS) and overall survival (OS), using the results of completed trials.
Abstract
Background: PARP inhibitors have been recently approved by the FDA for the treatment of metastatic castration-resistant prostate cancer (mCRPC). Their effectiveness is seen when used with androgen deprivation therapy in patients with or without deleterious germline and somatic genetic mutations.
Objectives: To identify all the randomized controlled trials (RCTs) in which PARP inhibitors have been assessed in the treatment of mCRPC, and to compare the efficacy of PARP inhibitors in these patients with standard-of-care (S/nonhormonal therapies like abiraterone acetate (Zytiga) or enzalutamide (Xtandi) in terms of progression-free survival (PFS) and overall survival (OS).
Search strategy: A systemic review search was conducted using PubMed, Embase, and Central Cochrane Registry.
Selection criteria: Randomized clinical trials with PARP inhibitors, with or without antihormonal therapy, as the intervention arm, with SOC as control.
Data analysis: HRs were calculated for PFS and OS. For effect sizes, a confidence interval of 95% was used, and for statistical significance, a P value of less than .05 was used. Analysis was done using random effects and fixed models and both were reported. Heterogeneity was evaluated using I2 statistic.
Results: Three RCTs were included in the analysis. PARP inhibitors showed a statistically significant improvement in OS when calculated using a fixed model (HR, 0.751; 95% CI, 0.582-0.968) but the improvement was not significant when calculated using a random model (HR, 0.758; 95% CI, 0.565-1.017; I2 = 23). Similarly, the improvement in PFS was statistically significant when calculated using a fixed model (HR, 0.626; 95% CI, 0.521-0.752), and no statistical significance was noted with a random model (HR, 0.674; 95% CI, 0.437-1.039; I2 = 80).
Conclusions: PARP inhibitors contributed to significant increases in PFS and OS when used with or without antihormonal agents like abiraterone or enzalutamide. This efficacy was pronounced among the patients with deleterious germline or somatic homologous recombination repair gene mutations, although patients without these mutations also showed a better PFS and OS in comparison with SOC therapy.
Oncology (Williston Park). 2021;35(11):708-715.
DOI: 10.46883/ONC.2021.3511.0708
Introduction
Prostate cancer is the second most common and fifth most aggressive cancer among men worldwide.1 According to one estimate, 1 in 7 US men and 1 in 25 men worldwide will be diagnosed with prostate cancer during his lifetime.1 Despite the advanced screening methods available, such as measurement of prostate-specific antigen levels, the incidence of metastatic disease remains as high as 20%.2 The best-known risk factors for prostate cancer are race (ie, African American descent), obesity, and genetics (eg, BRCA1/2 mutations). Gleason scoring is commonly used for histopathologic evaluation and for clinical and pathologic staging of disease. Patients with high-risk disease are treated with prostatectomy and/or external beam radiotherapy followed by androgen deprivation therapy (ADT) as maintenance therapy. If disease progression occurs while the patient is receiving ADT, the disease is noted to be castration-resistant prostate cancer (CRPC). Unfortunately, the majority of patients with prostate cancer progress to castration-resistant disease within 2 to 3 years.3
For decades the standard-of-care (SOC) treatment for metastatic CRPC (mCRPC) has been composed of cytotoxic agents, including taxanes (docetaxel or cabazitaxel [Jevtana]), and second-generation antihormonal agents (antihormonal therapy; AHT) such as abiraterone (Zytiga) or enzalutamide (Xtandi). Previously, CRPC was called “androgen-independent prostate cancer” and “hormone-refractory prostate cancer.”4 Subsequently, results of several studies showed that intratumoral (intracrine and paracrine) androgen production plays a significant role in the development of resistance among prostate cancer cells to testosterone suppression therapy.5
Other treatment options include pembrolizumab (Keytruda) for PD-L1–positive and microsatellite instability (MSI)–high disease, and radium-223 (Xofigo) for bone metastasis. PARP1 (or PARP) inhibitors are used in patients with mutations in homologous recombination repair (HRR) genes (most commonly, BRCA1/2). Recent study results indicate that the androgen receptor (AR) regulates the DNA repair pathways, and reciprocally, several enzymes involved in DNA repair can moderate AR activity.6-8 An example of such an enzyme is PARP1, which is involved in identifying single-stranded DNA breaks and their repair through the base excision method.9 Several cancers, including prostate cancer, exhibit increased PARP1 activity or expression.9-11 The mechanism of action of PARP inhibitors includes physical obstruction of the replication fork (PARP trapping), which affects HRR, resulting in DNA double-strand breaks.12 Previous study results have shown that these PARP inhibitors are synergistic when used with agents affecting the AR pathway regardless of HRR mutation status.9,13 In 2020, for the first time, the FDA approved PARP inhibitors for use in mCRPC.
The aim of this meta-analysis is to analyze the efficacy of these drugs in the treatment of mCRPC in terms of progression-free survival (PFS) and overall survival (OS), using the results of completed trials.
Methodology
The authors followed PRISMA guidelines.
Search Strategy
The databases accessed were Cochrane Central Registry of Clinical Trials, Embase, and PubMed. Search terms used were PARP inhibitors, prostate cancer, prostate neoplasm, olaparib (Lynparza), veliparib.
Inclusion and Exclusion Criteria
Papers had no restrictions in terms of date or status of
publications. To be included, however, the papers had to report on RCTs that:
1. compared PARP inhibitors against SOC in patients with mCRPC;
2. reported PFS and OS;
3. included only patients 18 years or older; and
4. were available in the English language.
Papers that did not meet the above criteria were excluded.
Trial Selection and Evaluation
Three authors independently reviewed all articles and abstracts and excluded the irrelevant trials. Risk of bias for selected papers was assessed using the Cochrane Collaboration tool and then classified as high, uncertain, or low.
Data Extraction
Information was extracted using a prespecified extraction table. Information was extracted from the papers by reading through the main texts and tables, and a second author reviewed the information collected to ensure its accuracy. The extracted data included HRs for PFS and OS.
Statistical Analysis
The meta-analysis was performed using Comprehensive Meta-analysis software version 3. HRs were calculated for PFS and OS . For effect sizes, a 95% CI was used, and a P value of less than .05 indicated statistical significance. Analysis was done using random and fixed models and both were reported. Heterogeneity was evaluated using I2 statistic and categorized as low (<40), moderate (40-60), and high (>60). Where a median was used, it was assumed to be equivalent to the mean, and SDs were estimated by dividing interquartile differences by 1.35. Fixed effect
analysis is usually adapted in cases where I2 value is ≤50%; otherwise, random effect model is used.
Literature Search
The initial search identified 351 articles; removal of duplicates left 322. The first screening excluded 262 articles. The full texts of the remaining 60 articles were analyzed. Thirty-seven articles were excluded because the trials described were incomplete; 13 were review articles; 2 described trials that were terminated; 4 were about single-arm studies; and 1 article’s study did not have relevant intervention. Ultimately, articles describing 3 RCTs were included, and these trials had a total of 682 patients. The PRISMA flow diagram is shown in Figure 1, and main characteristics of RCTs are listed in the Table.
FIGURE 1. PRISMA Flow Diagram for Meta-Analysis
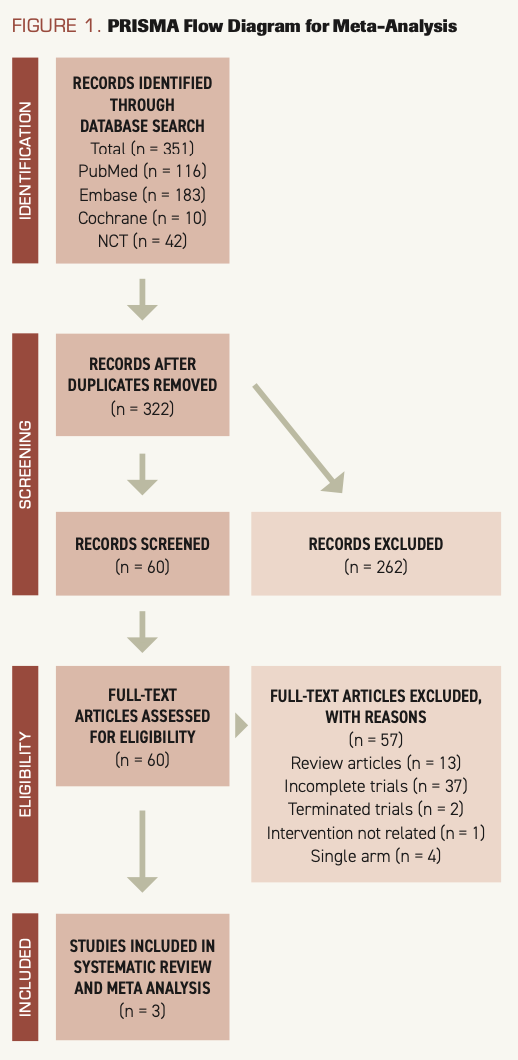
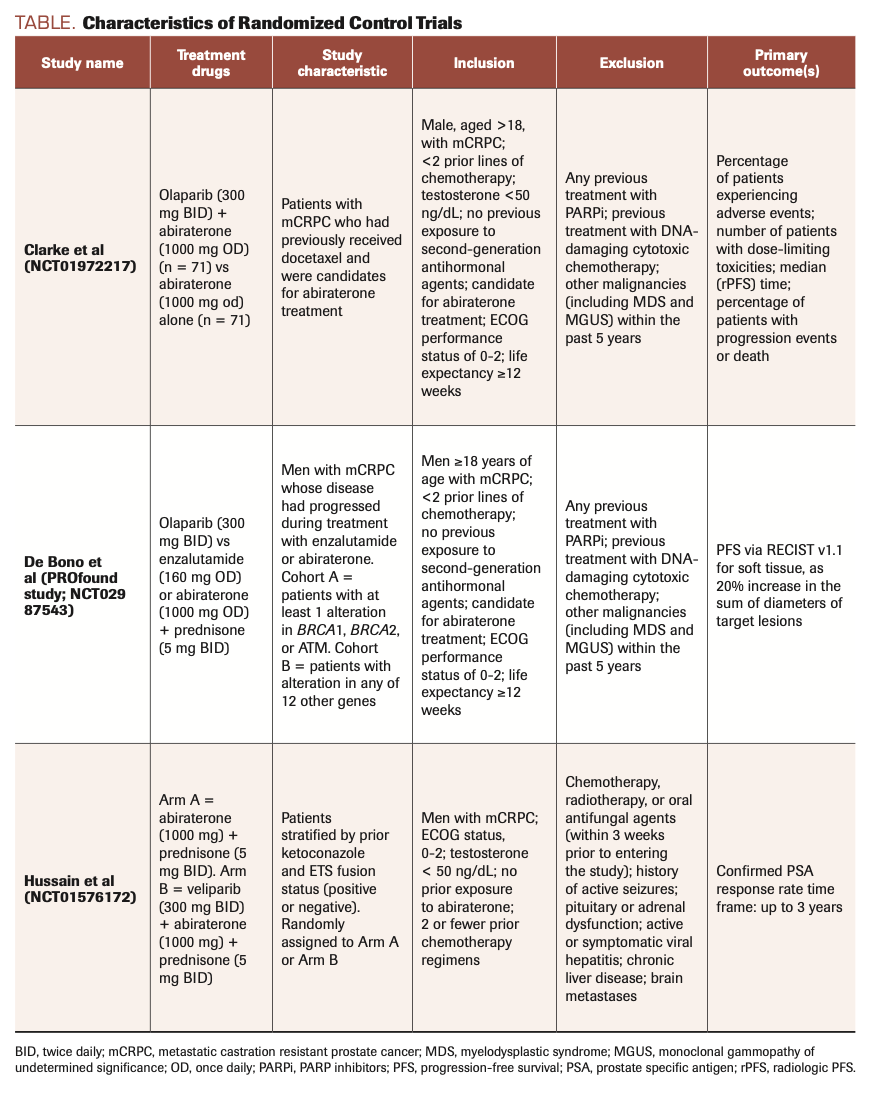
TABLE. Characteristics of Randomized Control Trials
Results
Risk of bias
The results of risk of bias are shown in Figure 2 and Figure 3.
FIGURE 2. Risk of Bias Graph: Review Authors’ Judgements About Each Risk of Bias Item Presented as Percentages Across All Included Studies
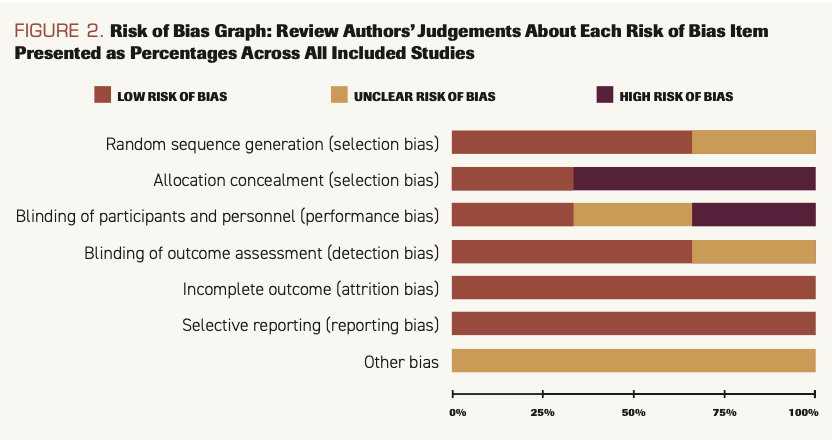
FIGURE 2. Risk of Bias Graph: Review Authors’ Judgements About Each Risk of Bias Item Presented as Percentages Across All Included Studies
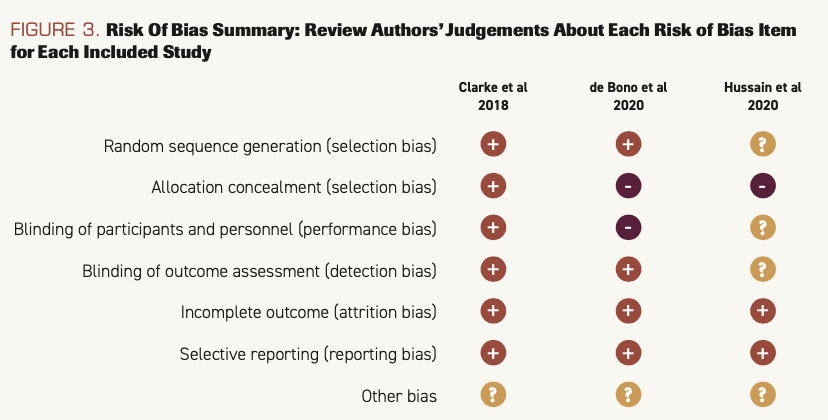
Results of quantitative analysis
Overall survival
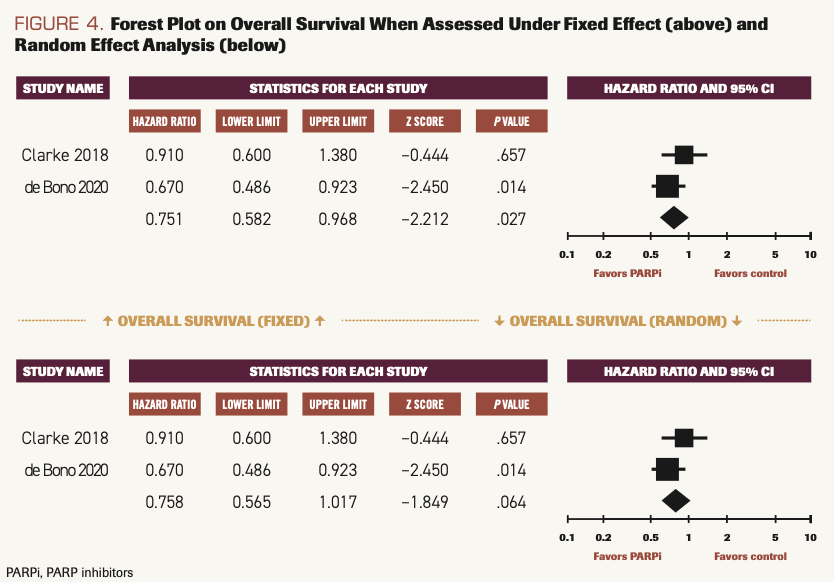
Two studies reported OS when patients used PARP inhibitors compared with SOC.14,15 The difference was statistically significant when calculated using the fixed model (HR, 0.751; 95% CI, 5.82-0.968; P = .027), and I2 = 23.23. When calculated using the random model, there was a strong deviation favoring PARP inhibitors, but it did not reach statistical significance (HR, 0.758; 95% CI, 0.565-1.017; P = .064) (Figure 4).
FIGURE 4. Forest Plot on Overall Survival When Assessed Under Fixed Effect (above) and Random Effect Analysis (below)
Progression-free survival
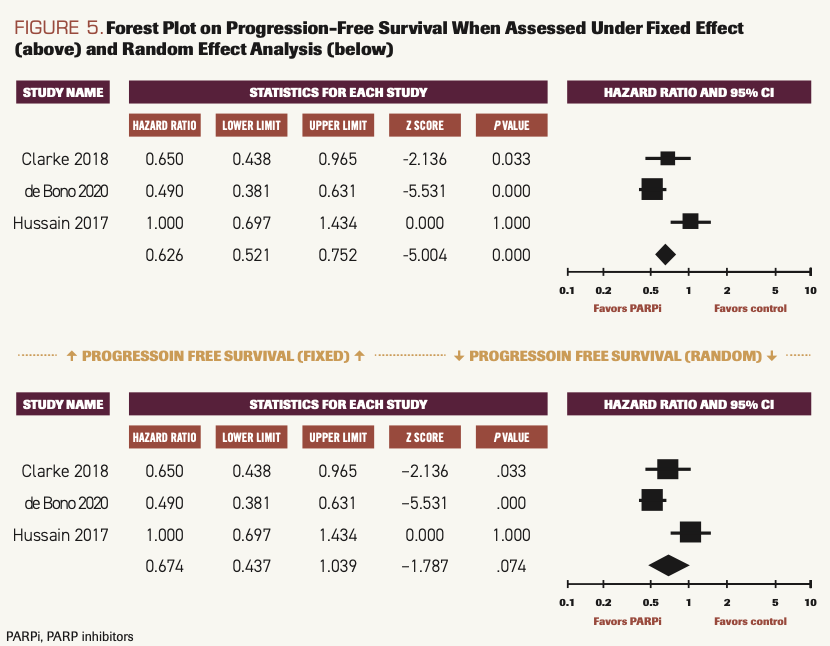
Three studies reported PFS when patients used PARP inhibitors compared with SOC.14-16 The difference was statistically significant when calculated using the fixed model (HR, 0.626; 95% CI, 0.521-0.752; P <.001), and I2 = 80.240. When calculated using the random model, there was a strong deviation favoring PARP inhibitors, but it did not reach statistical significance (HR, 0.674; 95% CI, 0.437-1.039; P = .074) (Figure 5).
FIGURE 5.Forest Plot on Progression-Free Survival When Assessed Under Fixed Effect (above) and Random Effect Analysis (below)
Discussion
Overall results of this meta-analysis indicate that patients with mCRPC experience survival benefits when treated with PARP inhibitors as compared with placebo or SOC chemotherapy. OS was better in the PARP inhibitor group under fixed effect (HR, 0.751; 95% CI,0.582-0.968; P = .027) and under random effect (HR, 0.758; 95% CI, 0.565-1.017; P = .064). PFS was improved in the PARP inhibitor group (HR, 0.626; 95% CI, 0.521-0.752; P <.001) compared with the chemotherapy group when analyzed under fixed effect.
In 2020, the FDA approved 2 PARP inhibitors—rucaparib (Rubraca) and olaparib—to treat mCRPC in patients harboring somatic and/or germline mutations in BRCA1 or BRCA2 as well as in ATM genes. This decision was based upon the data from the multicenter, single-arm TRITON2 clinical trial (NCT02952534), in which rucaparib was used in patients with mCRPC positive for BRCA mutations. Currently, numerous clinical trials are ongoing to determine the efficacy of PARP inhibitors in mCRPC. Ongoing clinical trials with olaparib, veliparib, rucaparib, niraparib (Zejula), and talazoparib (Talzenna) in mCRPC were searched on the clinicaltrials.gov website. Currently, 37 trials are ongoing, 2 have been terminated, and 3 trials have been completed.
In 2 trials, olaparib was the study drug.14,15 In Clarke et al, patients were randomized into the treatment group (abiraterone plus olaparib) or the control group (abiraterone alone) irrespective of any genetic mutations or biomarker criteria. The study showed statistically significant improvement in both PFS and OS in the treatment group, indicating that a broader population, regardless of HRR mutation status, can benefit from the synergy of PARP inhibitors and AR inhibitors. However, men with HRR mutations derive the greatest benefit from these medications.
Our literature review also shows that PARP inhibitors are much more effective in patients with HRR or ATM mutations. In the PROfound study (NCT02987543; de Bono et al), patients were divided into 2 cohorts: Cohort A included patients with 1 or more of 3 mutations (BRCA1/2; ATM), and Cohort B included patients with a mutation in any of 12 other prespecified genes. Each cohort was divided into a treatment arm (olaparib) and a control arm (enzalutamide or abiraterone). OS was prolonged when measured together for cohorts A and B: 17.5 months with PARP inhibitor vs 14.4 months with chemotherapy (HR for death, 0.67; 95% CI, 0.49-0.93). The PFS for cohort A vs cohort B was 7.4 months vs 3.6 months, respectively; HR for progression or death, 0.34; 95% CI, 0.25-0.47; P < .001. The results for Cohort A and B combined showed median PFS to be 5.8 months vs 3.5 months (HR, 0.49; 95% CI, 0.38-0.63; P <.001). Once again, these results indicate that PARP inhibitors are effective in patients with mCRPC regardless of genetic mutation status, although responses are much more evident in the population with BRCA and ATM alterations.
In the third trial (NCT01576172; Hussain et al), veliparib was compared with a control regimen of abiraterone plus prednisone.16 The investigators’ primary objective was to see if ETS fusion status (related to a family of transcription factors) has a role in the tumor response to the treatment. Patients were first divided according to ETS fusion status (positive or negative) and then equally distributed in the case and control cohorts. Surprisingly, there was no difference in PFS between the treatment arms, regardless of ETS status, with overall PFS of 11 months (95% CI, 8.1-13.6) in the treatment group vs 10.1 months (95% CI, 8.2-13.8) in the control group (P = .99). However, a significant finding was that DNA repair status (ie, DRD gene mutation) was associated with statistically significant improvement in PFS regardless of treatment status: 14.5 months (abnormal DRD gene) vs 8 months (normal DRD gene) (HR, 0.52; 95% CI, 0.29-0.93; P = .02). This study was not included in the forest plot for OS because OS was not calculated in this group.
Strengths and limitations
This study, as a meta-analysis, remains a retrospective chart review; the possibility for biases exists. Fewer trials and smaller study populations lead to publication bias. We made our best effort to locate all relevant published studies, randomize them, and complete data extraction and analysis. Another major limitation of this trial is the difficulty in performing stratified pool analysis for each PARP inhibitor drug; only a very limited number of completed phase 2/3 RCTs have currently available results. Other potential contributors to bias for this meta-analysis include heterogeneous population and inclusion criteria (with different first-line therapy patients and BRCA or other gene mutations). Another limitation is the inability to compare the adverse effect profiles between the PARP inhibitor and chemotherapy groups.
Conversely, a strength of this analysis is that it includes all phase 2/3 RCTs evaluating the efficacy of PARP inhibitors in mCRPC that have been completed and published, to date.
Conclusions
This meta-analysis shows that PARP inhibitors can prolong PFS or OS compared to SOC treatment in patients with mCRPC irrespective of HRR or other genetic mutation status. Longer PFS and OS were seen when PARP inhibitors were used alone or in combination with AHT therapies like abiraterone or enzalutamide. The effect was more significant when examined with a fixed model analysis. Although there was a significant deviation towards an increase in PFS and OS in the random model analysis, the effect was not statistically significant, and it was likely secondary to a relatively small patient population in the meta-analysis. Although, at baseline, there was heterogeneity among the populations participating in these trials, in terms of genetic alterations, the results of all the trials showed better outcomes in their intervention arms. This heterogeneity can be dealt with by incorporating more RCTs into meta-analyses going forward. More studies can further magnify these results once they are published.
DECLARATIONS
Ethics Approval and Consent to Participate: The data extracted and manuscript were reviewed with the Research Department and Ethics Committee of Department of Medicine, Staten Island University Hospital/Northwell. No experimental intervention was performed, and no specifications of guidelines, legislations, or permissions were required.
Availability of data and materials: Data are available in Excel files. Patient identifying information was removed at all stages in all the studies included.
Competing interests: No competing financial or personal interests are involved for all the authors.
Funding: No funding was obtained from any organization or personnel during any stage of manuscript writing or submission.
Author’s contributions: Manuscript written and data obtained by M.R.K.N, A.J, S.S, S.B.S. Proofreading and literature review done by D.A and A.B.
AUTHOR AFFILIATIONS:
1. Department of Internal Medicine, Zucker School of Medicine at Hofstra/Northwell at Staten Island University Hospital, Staten Island, NY, USA.
2. Division of Hematology and Medical Oncology, Zucker School of Medicine at Hofstra/Northwell at Staten Island University Hospital, Staten Island, NY, USA.
References
1. Barsouk A, Padala SA, Vakiti A, et al. Epidemiology, staging and management of prostate cancer. Med Sci (Basel). 2020;8(3):28. doi:10.3390/medsci8030028
2. Studer UE, Hauri D, Hanselmann S, et al. Immediate versus deferred hormonal treatment for patients with prostate cancer who are not suitable for curative local treatment: results of the randomized trial SAKK 08/88. J Clin Oncol. 2004;22(20):4109-4118. doi:10.1200/jco.2004.11.514
3. Harris WP, Mostaghel EA, Nelson PS, Montgomery B. Androgen deprivation therapy: progress in understanding mechanisms of resistance and optimizing androgen depletion. Nat Clin Pract Urol. 2009;6(2):76-85. doi:10.1038/ncpuro1296
4. Ahmad K. New progress in treatment of hormone-refractory prostate cancer. Lancet Oncol. 2004;5(12):706. doi:10.1016/s1470-2045(04)01641-9
5. Montgomery RB, Mostaghel EA, Vessella R, et al. Maintenance of intratumoral androgens in metastatic prostate cancer: a mechanism for castration-resistant tumor growth. Cancer Res. 2008;68(11):4447-4454. doi:10.1158/0008-5472.Can-08-0249
6. Haffner MC, Aryee MJ, Toubaji A, et al. Androgen-induced TOP2B-mediated double-strand breaks and prostate cancer gene rearrangements. Nat Genet. 2010;42(8):668-675. doi:10.1038/ng.613
7. Schiewer MJ, Knudsen KE. Linking DNA damage and hormone signaling pathways in cancer. Trends Endocrinol Metab. 2016;27(4):216-225 .doi:10.1016/j.tem.2016.02.004
8. Ta HQ, Gioeli D. The convergence of DNA damage checkpoint pathways and androgen receptor signaling in prostate cancer. Endocr Relat Cancer. 2014;21(5):R395-R407. doi:10.1530/erc-14-0217
9. Schiewer MJ, Goodwin JF, Han S, et al. Dual roles of PARP-1 promote cancer growth and progression. Cancer Discov. 2012;2(12):1134-1149. doi:10.1158/2159-8290.Cd-12-0120
10. Hirai K, Ueda K, Hayaishi O. Aberration of poly(adenosine diphosphate-ribose) metabolism in human colon adenomatous polyps and cancers. Cancer Res. 1983;43(7):3441-3446.
11. Fukushima M, Kuzuya K, Ota K, Ikai K. Poly(ADP-ribose) synthesis in human cervical cancer cell -diagnostic cytological usefulness. Cancer Lett. 1981;14(3):227-236. doi:10.1016/0304-3835(81)90148-8
12. O’Connor MJ. Targeting the DNA damage response in cancer. Mol Cell. 2015;60(4):547-560. doi:10.1016/j.molcel.2015.10.040
13. Asim M, Tarish F, Zecchini HI, et al. Synthetic lethality between androgen receptor signalling and the PARP pathway in prostate cancer. Nat Commun. 2017;8(1):374. doi:10.1038/s41467-017-00393-y
14. de Bono J, Mateo J, Fizazi K, et al. Olaparib for metastatic castration-resistant prostate cancer. N Engl J Med. 2020;382(22):2091-2102. doi:10.1056/NEJMoa1911440
15. Clarke N, Wiechno P, Alekseev B, et al. Olaparib combined with abiraterone in patients with metastatic castration-resistant prostate cancer: a randomised, double-blind, placebo-controlled, phase 2 trial. Lancet Oncol. 2018;19(7):975-986. doi:10.1016/s1470-2045(18)30365-6
16. Hussain M, Daignault-Newton S, Twardowski PW, et al. Targeting androgen receptor and DNA repair in metastatic castration-resistant prostate cancer: results from NCI 9012. J Clin Oncol. 2018;36(10):991-999. doi:10.1200/jco.2017.75.7310

Prolaris in Practice: Guiding ADT Benefits, Clinical Application, and Expert Insights From ACRO 2025
April 15th 2025Steven E. Finkelstein, MD, DABR, FACRO discuses how Prolaris distinguishes itself from other genomic biomarker platforms by providing uniquely actionable clinical information that quantifies the absolute benefit of androgen deprivation therapy when added to radiation therapy, offering clinicians a more precise tool for personalizing prostate cancer treatment strategies.