Guidelines for Palliative Treatment of Spinal Metastases: Choosing Between Stereotactic Body Radiation Therapy and Conventional Fractionation
ABSTRACT Symptomatic spinal metastasis is a frequent complication of cancer that had been treated, until relatively recently, with primitive techniques to modest radiation dose levels, with a baseline assumption of limited survival and poor patient performance in that setting. In the era of targeted and personalized therapies, many patients are living longer and more functionally and are able to manage their disease on the model of chronic illness. Given these developments, an attractive option is the use of stereotactic body radiation therapy (SBRT) to deliver high biologically effective doses of radiation conformally to maximize the palliative gains of treatment. However, randomized data to guide practice are scarce. We review the extant literature and present an algorithmic approach to selecting patients with metastatic disease for palliative spinal SBRT favoring the results of available randomized studies and remaining within the safety constraints supported by evidence from randomized trials.
Oncology (Williston Park). 2021;35(2):63-69.
DOI: 10.46883/ONC.2021.3502.0063
Eckstein is a resident physician in the Department of Radiation Medicine, Northwell Health Cancer Institute and Zucker School of Medicine.

Koffler is a resident physician in the Department of Radiation Medicine, Northwell Health Cancer Institute and Zucker School of Medicine.

Background
Spinal metastasis is noted in many common primary cancer sites such as breast, prostate, and lung, making palliation of symptomatic spinal metastases both a frequent and heterogeneous indication for radiation therapy.1
Historically, palliative radiation therapy for spinal disease was delivered using 2-dimensional (2D) planning techniques that delivered moderate doses of radiation to the involved portion of the spine over 5 to 15 treatments; beams coming from the anterior and posterior directions were placed and shaped using the location of involved spinal levels on a radiograph.2,3 Treatment with 2D technique is limited both by the detail provided by radiographs and the inability to spare the spinal cord and viscera located near the treatment target. Over time, the advent of CT and MRI, along with paradigm-shifting improvements in radiation planning and delivery technologies, facilitated the development of intensity modulated radiation therapy (IMRT). IMRT uses accurate 3D anatomical data, dynamic beam shaping, and inverse planning software to create radiation plans that treat the target to a desired dose and selectively spare organs at risk with millimeter-level accuracy.4 IMRT has made it possible to treat patients with ablative intent using larger doses in fewer treatments than ever before, a practice termed stereotactic body
radiotherapy (SBRT).
In palliation of spinal metastases, SBRT offers the prospect for improvements in pain palliation and local control with the delivery of ablative doses, making the use of this technique compelling to many radiation oncologists.5-8 Nevertheless, randomized data to guide the selection of spinal metastasis patients for SBRT versus conventionally fractionated external beam radiotherapy (EBRT) are sparse. While we await the reporting of further randomized data, the need for objective decision-making guidelines in this setting is pressing. We therefore present the following effort to synthesize the present literature into guidelines for radiation oncologists to prescribe treatment for spinal metastasis grounded in the best available evidence.
Narayana is a radiation oncologist in the Department of Radiation Medicine, Northwell Health Cancer Institute and Zucker School of Medicine.

Parashar is a radiation oncologist in the Department of Radiation Medicine, Northwell Health Cancer Institute and Zucker School of Medicine

This guideline is meant to guide the use of radiotherapy to treat spinal metastases only with palliative intent. If the patient is considered to be oligometastatic and meets criteria for recently published phase 2 trials9,10 in which ablative therapies have been suggested to prolong survival, their treatment should not be influenced by this guideline but rather by a decision focused around potential overall survival benefit.
Potters is chairman of the Department of Radiation Medicine, Northwell Health Cancer Institute and Zucker School of Medicine.

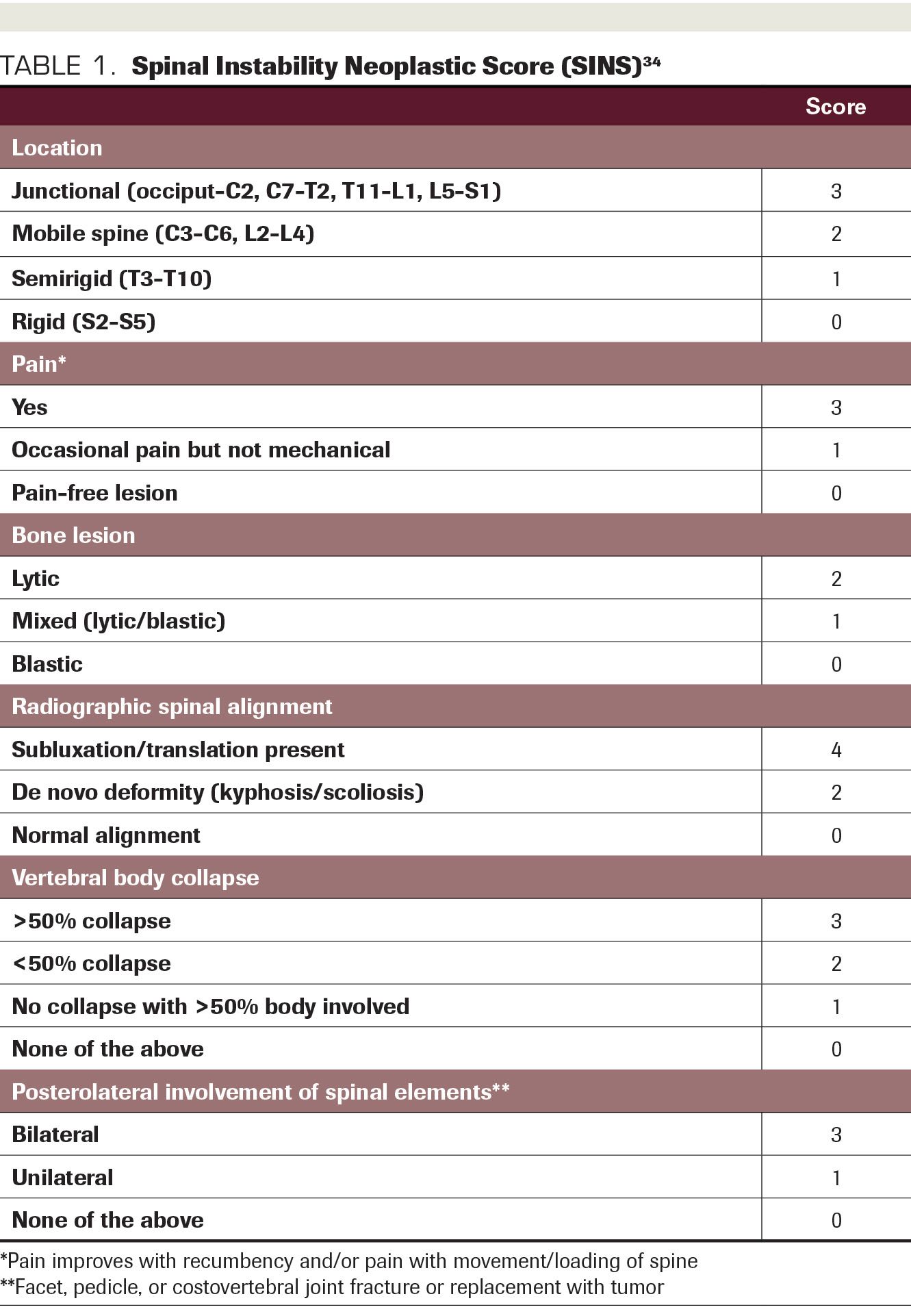
Additionally, spinal radiation should be delivered only after the necessity of prior surgical intervention has been ruled out. Neurosurgical consultation should be obtained if active neurological symptoms are present (due to myelopathy caused by malignant cord/cauda equina compression) or if significant mechanical instability exists, as assessed by the Spinal Instability Neoplastic Score (SINS)(Table 1); such instability could be exacerbated by radiotherapy-induced cytoreduction11.
Assuming that neurosurgical intervention has been diligently considered, the delivery of palliative radiation therapy for spinal metastases should be guided as follows:
TABLE 1. Spinal Instability Neoplastic Score
Will conventional palliative regimens (20 Gy in 5 fractions, 30 Gy in 10 fractions) provide durable control?
The likelihood of response to conventionally fractionated palliation for bone metastases is stratified by tumor histology.6,12 Primary tumor histologies for which conventional palliation is more likely to be successful in eliciting a significant tumor response include breast, ovarian, small cell, seminoma, and hematologic malignancies.13,14 For example, a Radiation Therapy Oncology Group (RTOG) study published in 1982 found superior pain relief in patients with breast and prostate histology compared with those with lung cancer.15 Moderately radioresistant histologies such as thyroid and colorectal cancers and non–small cell lung cancer (NSCLC) and, even more so, significantly radioresistant histologies such as sarcoma, melanoma, and renal cell carcinoma are unlikely to elicit favorable responses with EBRT.11,12 Radioresistant histologies include sarcoma, melanoma, renal cell carcinoma, thyroid and colorectal cancers, and NSCLC.6,12-14 Patients with favorable histology are more likely to remain ambulatory for longer periods of time and have good pain response after conventional radiation therapy.12,16
Because conventional palliation will likely provide poor results in radioresistant histologies, is SBRT a better option?
The poor outcomes with conventional palliation in radioresistant spinal metastases have led to a shift in practice toward SBRT (16-24 Gy in 1 fraction, 24-30 Gy in 3 fractions) for these lesions. These fractionation schemes result in an enhanced biologically effective dose (BED), double-stranded DNA breaks, immunogenicity, and vascular supply disruption relative to conventional fractionation.17,18 Multiple patient series6-8 and a randomized phase 2 trial5 have shown that these biological differences translate into improved clinical local control and pain relief when SBRT is used for spinal metastases, with local control at 24 months post treatment ranging from 84% to 98%.6-8 A phase 2/3 randomized trial investigated 16 Gy in 1 fraction compared with 8 Gy in 1 fraction with a primary end point of pain control at 3 months, and although the phase 2 portion of the study found 16 Gy/1 fraction to be safe,19 the phase 3 portion’s preliminary results reported at the 2019 national meeting of the American Society for Radiation Oncology did not find any improvement in pain control at 3 or 6 months after treatment. Although 3-month pain control is felt to be an important end point, previously mentioned data sets suggest that SBRT is more likely to provide durable pain and local control over time.
Importantly, the available randomized data select a primary end point of pain relief at 3 months post treatment.19 As metastatic cancer is fundamentally a systemic disease, this end point provides for a good balance between optimization of quality of life and expected survival. Pain relief at 6 months, prevention of neurological events, etc, are legitimate secondary end points, but they should not supervene upon the 3-month end point in terms of guiding treatment decisions. Likewise, outside of the context of treatment for control of oligometastatic disease, the theoretical biological prospect of improved local control at the treated site is not helpful in guiding management of patients who are overwhelmingly likely to develop further spinal disease progression.
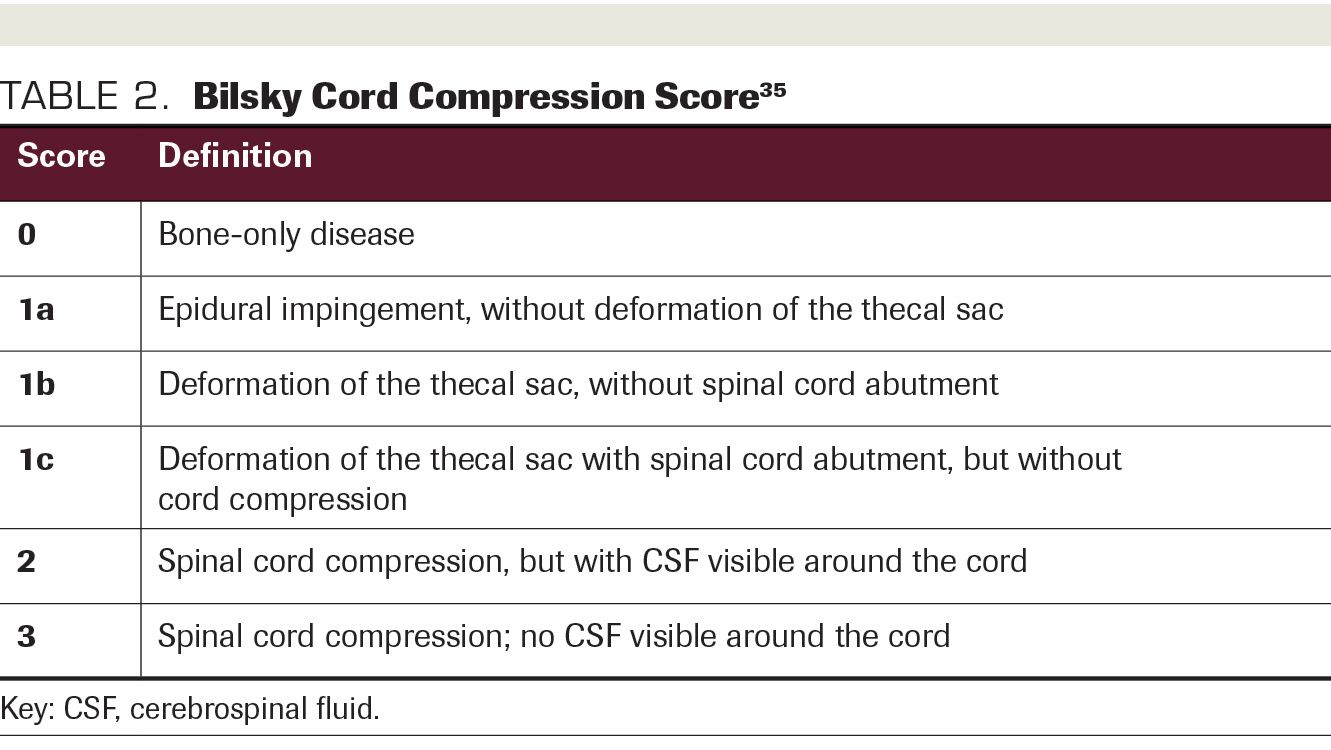
TABLE 2. Bilsky Cord Compression Score
Could weaknesses of RTOG 0631 underrepresent the therapeutic potential of SBRT with regard to its primary end point of short-term pain control?
In addition to the limited follow-up in RTOG 0631, which raises questions about the durability of the pain response demonstrated by the 8-Gy arm, some have suggested that 16 Gy may be an inadequate single-fraction dose to maximize the benefit of disease control.20 Sprave et al reported in a randomized phase 2 trial that utilizing 24 Gy in 1 fraction compared with 30 Gy in 10 fractions did show that complete pain response in the SBRT group was significantly more likely at 6 months (53% vs 10%; P = .0034) and trended toward significance at 3 months (43% vs 17%; P = .0568).5 Because 30 Gy in 10 fractions was deemed to be equivalent to 8 Gy in 1 fraction with respect to pain control in a randomized phase 3 trial,21 the phase 2 data from Sprave et al showing superiority of 24 Gy/1 fraction over 30 Gy/10 fractions in conjunction with RTOG 0613 reporting equivalency of 16 Gy/1 to 8 Gy/1 suggest 16 Gy may be an inadequate single fraction dose for maximal pain response.
This concern is bolstered by a retrospective series by Yamada et al consisting of 657 patients and 811 lesions; the results indicated inferior local control when the dose received by 95% of the gross tumor volume (GTV D95) was less than 18.3 Gy.8 Although the entire cohort experienced an impressive local failure rate of only 3.1% at 48 months, those who received GTV D95 < 18.3 Gy had a failure rate of 14% and those with a GTV D95 >18.3 Gy had a failure rate of 2.5%.8 A larger SBRT dose is being employed in a phase 3 trial (CCTG-SC24) run by the Canadian Cancer Trial Group, which is comparing 24 Gy/2 fractions with 20 Gy/5 fractions in the treatment of spinal metastases, with a primary outcome of 3-month pain control. Assuming an alpha/beta ratio of 10, BED10 of the SBRT regimen used in CCTG-SC24 (52.80 Gy) is similar to the dose cut point for superior local control described by Yamada et al (51.79 Gy) and significantly larger that the BED of the dose used in SBRT arm of RTOG 0613 (41.6 Gy). In summary, we cannot generalize the results of RTOG 0631 to SBRT with higher doses, and recently closed phase 3 trials such as CCTG-SC24 will help the community decide if SBRT dose escalation can improve pain control beyond what is possible with conventional fractionation.
Despite the previously mentioned concerns grounded in retrospective and phase 2 evidence, RTOG 0613 has supplied the only randomized phase 3 evidence that compares SBRT with conventional palliation of spine metastases. Its 3-month pain response end point minimizes risk of confounding in pain reporting due to new symptomatic metastases and allows adequate time for cytoreduction and pain response. Additionally, 16 Gy, in contrast with 24 Gy, is a dose that can be delivered in a single fraction without invasive cord localization procedures such as CT myelogram, which many institutions will not have available. Thus, until we have results of other phase 3 trials, such as CCTG-SC24, RTOG 0631 should guide clinical practice for all patients in whom 3-month or 6-month pain control is the outcome of interest.
What are the limits of eligibility for SBRT with respect to spinal disease burden and proximity of disease to the cord?
In contrast with conventional fractionation, which can be safely delivered over multiple dose levels simultaneously using a 2D setup with acceptance of planning target volume (PTV) dose spill into the spinal cord, SBRT must be delivered with meticulous motion control and IMRT planning in order to enable ablative dose delivery to the vertebrae while avoiding the cord. Despite advancements in treatment planning and high conformality achieved by these techniques, concern remains about treating disease with high-risk characteristics such as epidural extension, cord compression, paraspinal extension, and multiple consecutive vertebrae involved. For example, the results of RTOG 0613 deemed any lesion within 3 mm of the spinal cord ineligible for randomization, although lesions with Bilsky 1a and 1b cord compression (Table 2) were still permitted as long as they were at least 3 mm away from the cord. Additionally, no more than 2 consecutive vertebrae could be included. In CCTG-SC24, patients with up to 3 consecutive vertebrae requiring treatment were eligible for randomization, and no distance requirement was set forth with respect to cord compression. Rather than distance, neurologic deficit (eg, motor, bowel, bladder dysfunction) due to cauda equina syndrome or cord compression was used as an exclusion criterion.22 With respect to paraspinal extension, RTOG restricted the size of paraspinal lesions to no larger than 5 cm, while CCTG does not have a restriction for paraspinal extension.
The previously mentioned high-risk features increase the risk of excessive doses of radiation to the spinal cord and other regional organs at risk (OARs), due to close proximity of disease to the cord or increased volume of disease (eg, epidural extension, cord compression, multiple consecutive vertebrae treated), increased dose spill into other important regional OARs (eg, large-volume paraspinal extension and multiple consecutive vertebrae), and inadequate setup accuracy (eg, multiple consecutive vertebrae in a field). Additionally, cord compression has been associated with increased risk for local relapse. In a series of 98 spinal lesions including 60 with epidural extension treated with multifraction SBRT, Mehta et al reported that Bilsky grade trended toward increased risk of local failure (P = .09).23 Despite epidural extension being present in 61% of lesions, the patients in that series experienced a local control rate of 93% at 6 months and 84% at 1 year. Toxicity was also well controlled, with 4.2% of treated patients experiencing vertebral body fracture and no reported radiation myelopathy.23 Kowalchuk et al evaluated high-risk treatments—defined as those including at least 2 consecutive vertebrae, epidural involvement of the tumor, or paraspinal soft tissue involvement of the tumor—in 54 patients with 62 lesions. All lesions were treated with 24 Gy in 3 fractions, and at a median follow-up of 14.36 months, local failure was only 8% and pain had improved in 76% of patients. Toxicity outcomes were excellent, with no cases of myelopathy and no new vertebral fractures of the treated vertebrae.24 The outcomes in these series, along with the recommendations of other recently published reviews,20,25 indicate that treating with a hypofractionated approach is an effective and safe accommodation for patients with paraspinal extension, moderate cord compression (Bilsky 1a, 1b, and occasionally 1c), and involvement of multiple consecutive vertebral levels. These retrospective series indicate that there may be room for expanding the eligibility for SBRT in the future, but at this time there are not phase 3 data indicating safety for SBRT to spinal metastases that don’t meet the RTOG 0631 eligibility criteria.19
How many contiguous spinal levels can be treated with SBRT?
RTOG 0631 restricted SBRT to at most 2 contiguous spinal levels.14 Chief concerns in the application of SBRT to disease that occupies 3 or more spinal levels include exacerbated setup error and toxicity to adjacent long OARs such as the spinal cord and esophagus. Experience has shown that numerous centers do prescribe SBRT to multilevel spinal disease. Wang et al, as noted above, have shown that concerns of setup error can be alleviated by appropriate immobilization and utilization of a 6D couch, such that as many as 9 contiguous levels can be treated without exceeding 1-mm residual error. However, these findings do not apply above the level of T2, where natural spinal curvature amplifies setup risks, and we consequently counsel caution in the treatment of bulky disease superior to T2. Well-established protocols have been accepted to treat, for example, central lung tumors to significantly greater BED10 than is given in spine SBRT, as per RTOG 0813. We defer to the constraints used in such protocols and suggest that treatment of multilevel disease should not be restricted by concern for normal tissue dose, provided these constraints can be met. Conversely, SBRT should not be utilized when coverage goals cannot be met without violating
those constraints.
When treating consecutive levels, bulky lesions, or lesions with epidural extension, how can we ensure the safety of treatment with respect to OARs if treating with SBRT compared with conventional palliation?
As with all radiation treatments, OAR dose must be kept below the established organ-specific constraints to ensure safety. Accurate contouring of OARs is important for accurate dose calculation. Additionally, if the patient requires more radiation in the future, an understanding of the cumulative dose received across multiple treatments will be necessary to build a safe treatment plan. The proper constraint for a given OAR depends on the number of fractions used, due to changes in the BED-dependent fraction size. One of the first sets of guidelines about safe OAR constraints in SBRT was published by Timmerman et al in 2008.26 These were followed shortly after by new but similar guidelines in 2010 from the American Academy of Physicists in Medicine (AAPM), which published the TG 101 report,27 and the Quantitative Analysis of Normal Tissue Effects in the Clinic (QUANTEC) group effort in 2010.28 As an update to the AAPM TG 101 dose constraint guidelines, Saghal et al published an updated set of recommendations for spinal cord constraints in 2019.29 At our institution, we follow TG 101 guidelines for OAR constraints during treatment of spinal metastases with SBRT.
Although the TG 101 and QUANTEC guidelines report constraints for the cord itself, many physicians choose to account for movement of the cord within the spinal canal by adding a 1- to 3-mm margin around the cord to create a planning OAR volume (PRV). Alternatively, the avoidance structure can be defined as the thecal sac, which applies an anatomically defined margin of approximately 1.5 mm beyond the spinal cord. In this case, the size of the margin varies among spinal segments, with the largest typically in the upper cervical spine.29 It should be noted that when reviewing OAR constraints in literature, the presence and size of PRV margin varies by report, which makes aggregating data and drawing conclusions from the literature as a whole difficult. These inconsistencies among reports were acknowledged in the 2019 guidelines published by Saghal et al, and they proposed reporting standards for subsequent case series evaluating radiation myelitis.
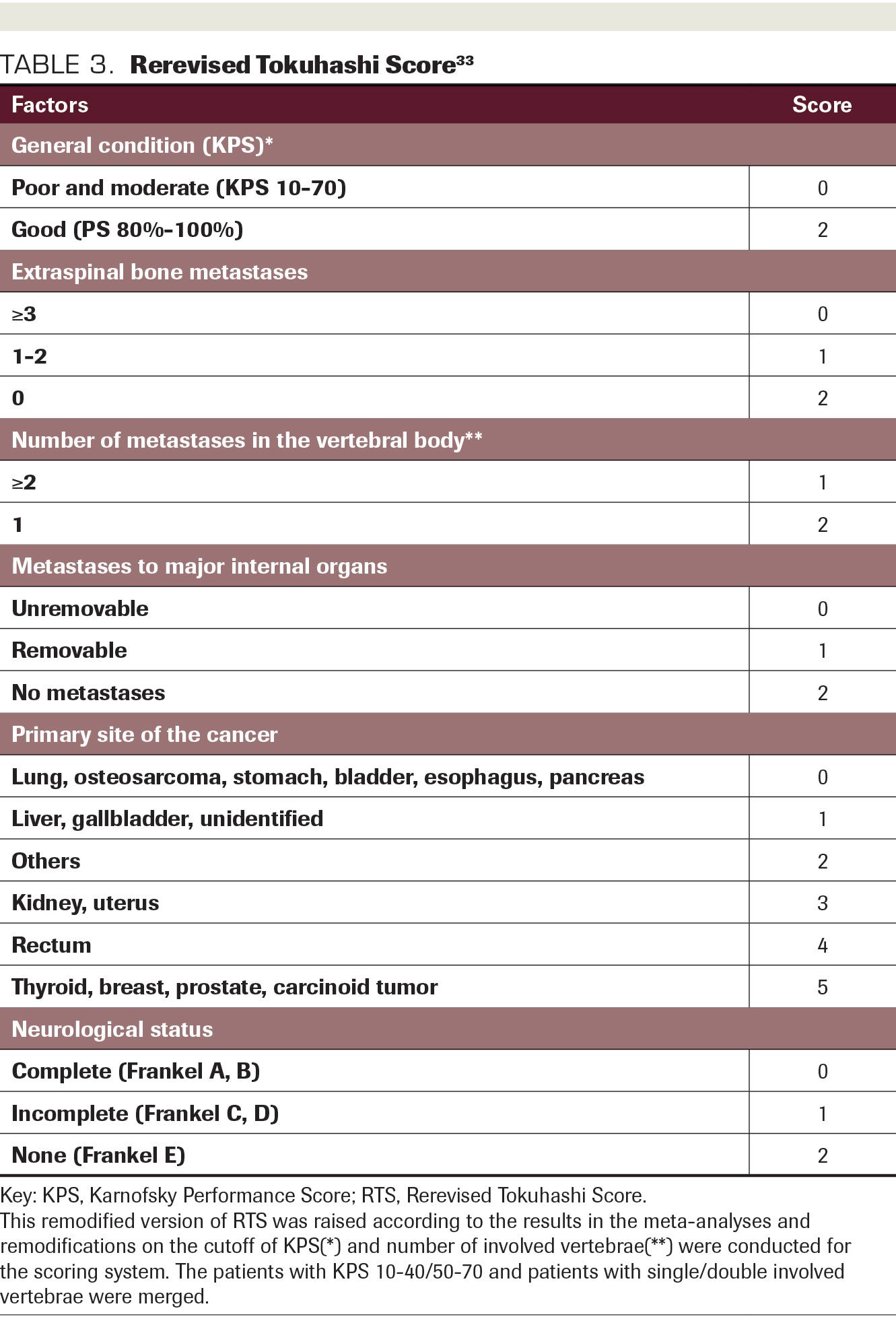
TABLE 3. Rerevised Tokuhashi Score
Furthermore, we propose that PTV volume and OAR definitions in spinal SBRT should adhere strictly to the RTOG guidelines governing the available randomized data. Practically speaking, this means that an inability to meet requirements for both clinical target volume/PTV coverage and OAR constraints should rule out the use of SBRT, at least until such time as more liberal guidelines are validated in a randomized trial. In particular, RTOG makes use of the MRI-defined spinal cord as an OAR, and this should not be substituted with a spinal canal OAR or replaced with a non–MRI-defined structure. Likewise, given the available safety data, SBRT reirradiation of volumes previously treated with conventional EBRT, but not volumes previously treated with SBRT, is an option.14
Recommendations
The following recommendations are presented algorithmically in the Figure. Because 3-month pain control has been shown to be noninferior to conventional palliation and we have reasonable suspicion to believe that long-term local control and pain control might be better with SBRT, the proper selection of palliative therapy for patients with radioresistant histology depends on their expected survival. Multiple survival scoring systems have been proposed for patients with spinal metastases including the revised Tokuhashi score,30 the Bauer modified score,31 and the Tomita score.32 These survival scores are all greater than 10 years old and lag behind the previous decade’s innovations in systemic therapy, surgery, and radiotherapy; thus, they may underestimate survival. Of the above scoring systems, the Tokuhashi scale was subject to a robust systematic review and meta-analysis published in 2018,33 and for that reason we defer to the rerevision of the Tokuhashi score in adducing patients’ estimated survival (Table 3).33
FIGURE. Spinal SBRT vs ERBT Decision Tree
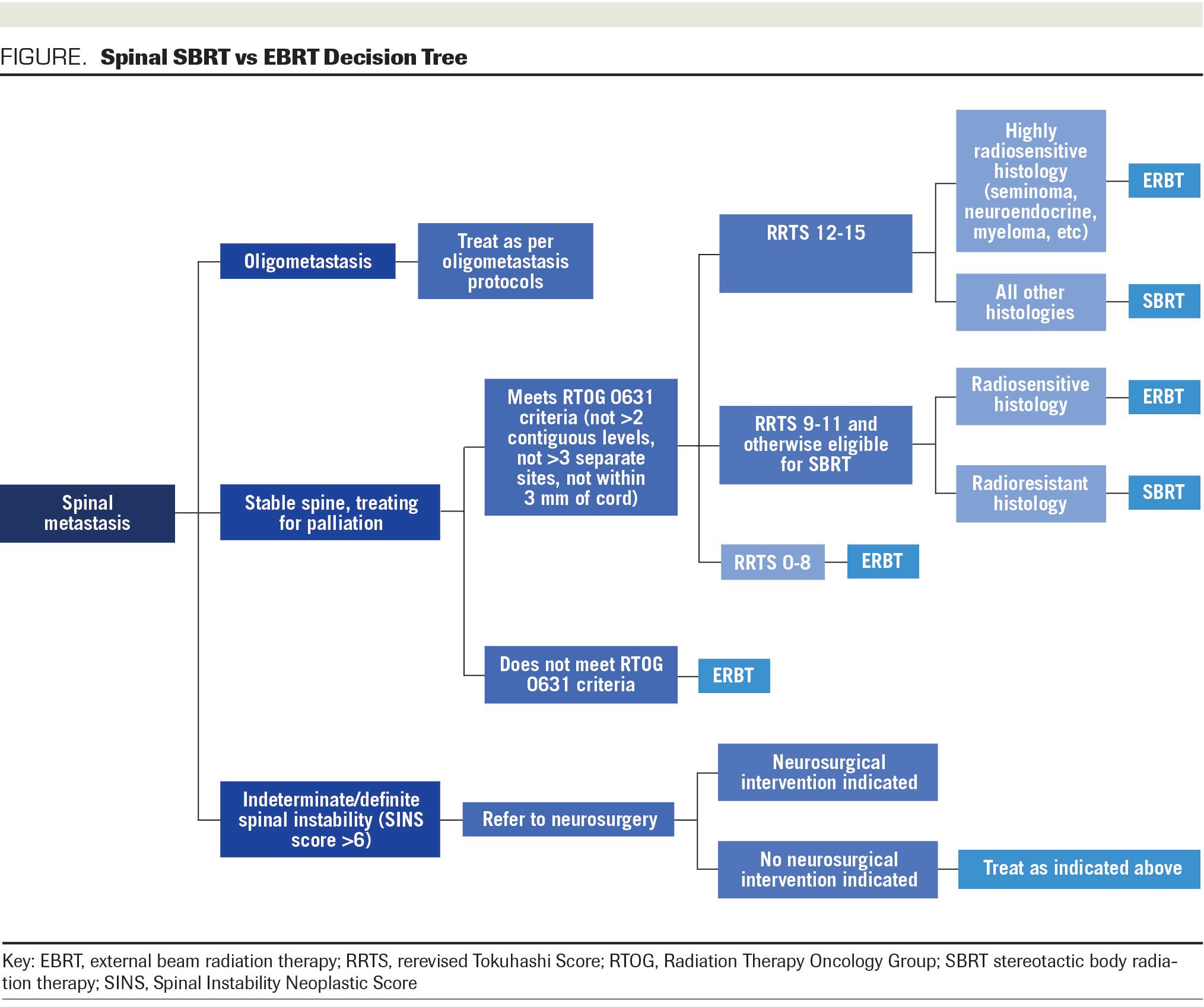
Patients with oligometastatic disease being treated with the aim of durable long-term control and possible improved survival based upon promising findings with the use of SBRT are outside and beyond the scope of these guidelines.
Initial consideration should be made for surgical evaluation. Patients with definite or indeterminate spinal instability, defined as a SINS score >6, should be referred to neurosurgery prior to radiotherapeutic decision-making and considered for RT only upon clearance from neurosurgery.
Patients with a stable spine and a treatment aim of palliation can be considered for SBRT. Nevertheless, those who do not meet RTOG 0631 eligibility criteria should be treated with conventional EBRT. For those with disease in more than 2 consecutive vertebrae per site, >3 nonconsecutive sites, or any disease within 3 mm of the spinal cord, conventional radiotherapy is recommended at this time, because RTOG 0631 excluded patients outside these parameters and safety and efficacy data do not currently support exceeding these constraints.19
In patients who meet RTOG 0631 criteria, we stratify by survival. For patients with estimated survival less than 6 months, equivalent to a rerevised Tokuhashi score (RRTS) of 0-8, it is likely that 3-month pain control may be the most relevant outcome, so we recommend conventional palliation for initial treatment regardless of histology.
For patients with estimated survival greater than or equal to 1 year, equivalent to RRTS 12 to15, we favor an SBRT approach unless contraindicated by other considerations (such as highly radiosensitive histology such as seminoma, myeloma, lymphoma, or neuroendocrine tumor).
For patients with estimated survival between 6 months and 1 year, equivalent to RRTS 9 to11, careful individualized selection of candidates for SBRT is favored. Radioresistant histology in such cases weighs in favor of offering SBRT and radiosensitive histology weighs against it.
Patients who progress after an initial course of conventional radiotherapy can be reirradiated by either conventional or SBRT technique, and the foregoing algorithm applies mutatis mutandis. However, SBRT is not an option for reirradiation of volumes previously treated by SBRT, regardless of other considerations, until safety data from randomized trials are available to validate this application of SBRT.
Financial Disclosure: The authors have no significant financial interest in or other relationship with the manufacturer of any product or provider of any service mentioned in this article.
REFERENCES
1. Maccauro G, Spinelli MS, Mauro S, Perisano C, Graci C, Rosa MA. Physiopathology of spine metastasis. Int J Surg Oncol. 2011;2011:107969. doi:10.1155/2011/107969
2. Herrmann H, Seppenwoolde Y, Georg D, Widder J. Image guidance: past and future of radiotherapy. Radiologe. 2019;59(suppl 1):21-27. doi:10.1007/s00117-019-0573-y
3. Høyer M, Thor M, Thörnqvist S, Søndergaard J, Lassen-Ramshad Y, Paul Muren L. Advances in radiotherapy: from 2D to 4D. Cancer Imaging. 2011;11 Spec No A(1A):S147-S152. doi:10.1102/1470-7330.2011.9036
4. Cho B. Intensity-modulated radiation therapy: a review with a physics perspective. Radiat Oncol J. 2018;36(1):1-10. doi:10.3857/roj.2018.00122
5. Sprave T, Verma V, Förster R, et al. Randomized phase II trial evaluating pain response in patients with spinal metastases following stereotactic body radiotherapy versus three-dimensional conformal radiotherapy. Radiother Oncol. 2018;128(2):274-282. doi:10.1016/j.radonc.2018.04.030
6. Gerszten PC, Mendel E, Yamada Y. Radiotherapy and radiosurgery for metastatic spine disease: what are the options, indications, and outcomes? Spine (Phila Pa 1976). 2009;34(22 suppl):S78-S92. doi:10.1201/9781315154053-7
7. Guckenberger M, Mantel F, Gerszten PC, et al. Safety and efficacy of stereotactic body radiotherapy as primary treatment for vertebral metastases: a multi-institutional analysis. Radiat Oncol. 2014;9:226. doi:10.1186/s13014-014-0226-2
8. Yamada Y, Katsoulakis E, Laufer I, et al. The impact of histology and delivered dose on local control of spinal metastases treated with stereotactic radiosurgery. Neurosurg Focus. 2017;42(1):E6. doi:10.3171/2016.9.FOCUS16369
9. Gomez DR, Tang C, Zhang J, et al. Local consolidative therapy vs. maintenance therapy or observation for patients with oligometastatic non–small-cell lung cancer: long-term results of a multi-institutional, phase II, randomized study. J Clin Oncol. 2019;37(18):1558-1565. doi:10.1200/JCO.19.00201
10. Palma DA, Olson R, Harrow S, et al. Stereotactic ablative radiotherapy versus standard of care palliative treatment in patients with oligometastatic cancers (SABR-COMET): a randomised, phase 2, open-label trial. Lancet. 2019;393(10185):2051-2058. doi:10.1016/S0140-6736(18)32487-5
11. Barzilai O, Laufer I, Yamada Y, et al. Integrating evidence-based medicine for treatment of spinal metastases into a decision framework: neurologic, oncologic, mechanicals stability, and systemic disease. J Clin Oncol. 2017;35(21):2419-2427. doi:10.1200/JCO.2017.72.7362
12. Maranzano E, Latini P. Effectiveness of radiation therapy without surgery in metastatic spinal cord compression: final results from a prospective trial. Int J Radiat Oncol Biol Phys. 1995;32(4):959-967. doi:10.1016/0360-3016(95)00572-g
13. Rades D, Fehlauer F, Schulte R, et al. Prognostic factors for local control and survival after radiotherapy of metastatic spinal cord compression. J Clin Oncol. 2006;24(21):3388-3393. doi:10.1200/JCO.2005.05.0542
14. Rades D, Fehlauer F, Stalpers LJ, et al. A prospective evaluation of two radiotherapy schedules with 10 versus 20 fractions for the treatment of metastatic spinal cord compression: final results of a multicenter study. Cancer. 2004;101(11):2687-2692. doi:10.1002/cncr.20633
15. Tong D, Gillick L, Hendrickson FR. The palliation of symptomatic osseous metastases: final results of the study by the Radiation Therapy Oncology Group. Cancer. 1982;50(5):893-899. doi:10.1002/1097-0142(19820901)50:5<893::AID-CNCR2820500515>3.0.CO;2-Y
16. Katagiri H, Takahashi M, Inagaki J, et al. Clinical results of nonsurgical treatment for spinal metastases. Int J Radiat Oncol Biol Phys. 1998;42(5):1127-1132. doi:10.1016/S0360-3016(98)00288-0
17. Vignard J, Mirey G, Salles B. Ionizing-radiation induced DNA double-strand breaks: a direct and indirect lighting up. Radiother Oncol. 2013;108(3):362-369. doi:10.1016/j.radonc.2013.06.013
18. Garcia-Barros M, Paris F, Cordon-Cardo C, et al. Tumor response to radiotherapy regulated by endothelial cell apoptosis. Science. 2003;300(5622):1155-1159. doi:10.1126/science.1082504
19. Ryu S, Pugh SL, Gerszten PC, et al. RTOG 0631 phase 2/3 study of image guided stereotactic radiosurgery for localized (1-3) spine metastases: phase 2 results. Pract Radiat Oncol. 2014;4(2):76-81. doi:10.1016/j.prro.2013.05.001
20. Osborn VW, Lee A, Yamada Y. Stereotactic body radiation therapy for spinal malignancies. Technol Cancer Res Treat. 2018;17:1533033818802304-1533033818802304. doi:10.1177/1533033818802304
21. Hartsell WF, Scott CB, Watkins Bruner D, et al. Randomized trial of short- versus long-course radiotherapy for palliation of painful bone metastases. J Natl Cancer Inst. 2005;97(11):798-804. doi:10.1093/jnci/dji139
22. Sahgal A, Myrehaug S, Dennis K, et al. A randomized phase II/III study comparing stereotactic body radiotherapy (SBRT) versus conventional palliative radiotherapy (CRT) for patients with spinal metastases (NCT02512965). J Clin Oncol. 2017;x(x):x-x. doi:10.1200/jco.2017.35.15_suppl.tps10129
23. Mehta N, Zavitsanos PJ, Moldovan K, et al. Local failure and vertebral body fracture risk using multifraction stereotactic body radiation therapy for spine metastases. Adv Radiat Oncol. 2018;3(3):245-251. doi:10.1016/j.adro.2018.04.002
24. Kowalchuk RO, Waters MR, Martin Richardson K, et al. Stereotactic radiosurgery for the treatment of bulky spine metastases. J Neurooncol. 2020;148(2):381-388. doi:10.1007/s11060-020-03534-4
25. Zeng KL, Tseng CL, Soliman H, Weiss Y, Sahgal A, Myrehaug S. Stereotactic body radiotherapy (SBRT) for oligometastatic spine metastases: an overview. Front Oncol. 2019;9(x):1-11. doi:10.3389/fonc.2019.00337
26. Timmerman RD. An overview of hypofractionation and introduction to this issue of Seminars in Radiation Oncology. Semin Radiat Oncol. 2008;18(4):215-222. doi:10.1016/j.semradonc.2008.04.001
27. Benedict SH, Yenice KM, Followill D, et al. Stereotactic body radiation therapy: the report of AAPM Task Group 101. Med Phys. 2010;37(8):4078-4101. doi:10.1118/1.3438081
28. Kirkpatrick JP, van der Kogel AJ, Schultheiss TE. Radiation dose-volume effects in the spinal cord. Int J Radiat Oncol Biol Phys. 2010;76(3 suppl):S42-S49. doi:10.1016/j.ijrobp.2009.04.095
29. Sahgal A, Chang JH, Ma L, et al. Spinal cord dose tolerance to stereotactic body radiation therapy. Int J Radiat Oncol Biol Phys. Published online October 10, 2019. doi:10.1016/j.ijrobp.2019.09.038
30. Tokuhashi Y, Matsuzaki H, Oda H, Oshima M, Ryu J. A revised scoring system for preoperative evaluation of metastatic spine tumor prognosis. Spine (Phila Pa 1976). 2005;30(19):2186-2191. doi:10.1097/01.brs.0000180401.06919.a5
31. Bauer HC, Wedin R. Survival after surgery for spinal and extremity metastases: prognostication in 241 patients. Acta Orthop Scand. 1995;66(2):143-146. doi:10.3109/17453679508995508
32. Tomita K, Kawahara N, Kobayashi T, Yoshida A, Murakami H, Akamaru T. Surgical strategy for spinal metastases. Spine (Phila Pa 1976). 2001;26(3):298-306. doi:10.1097/00007632-200102010-00016
33. Yang X-G, Lun D-X, Hu Y-C, et al. Prognostic effect of factors involved in revised Tokuhashi score system for patients with spinal metastases: a systematic review and meta-analysis. BMC Cancer. 2018;18(1):1248. doi:10.1186/s12885-018-5139-2
34. Fisher CG, DiPaola CP, Ryken TC, et al. A novel classification system for spinal instability in neoplastic disease: an evidence-based approach and expert consensus from the Spine Oncology Study Group. Spine (Phila Pa 1976). 2010;35(22):E1221-E1229. doi:10.1097/BRS.0b013e3181e16ae2
35. Bilsky MH, Laufer I, Fourney DR, et al. Reliability analysis of the epidural spinal cord compression scale. J Neurosurg Spine. 2010;13(3):324-328. doi:10.3171/2010.3.SPINE09459

Oncology Peer Review On-The-Go: Cancer-Related Fatigue Outcome Measures in Integrative Oncology
September 20th 2022Authors Dori Beeler, PhD; Shelley Wang, MD, MPH; and Viraj A. Master, MD, PhD, spoke with CancerNetwork® about a review article on cancer-related fatigue published in the journal ONCOLOGY®.
