Utility of the 21-Gene Recurrence Score in Node-Positive Breast Cancer
ABSTRACT The 21-gene Recurrence Score (RS) assay has been validated as both a prognostic and predictive tool in node-negative (pN0), estrogen receptor–positive (ER+), HER2-negative (HER2–) breast cancer. A large body of evidence supports the clinical utility of the RS in the node positive (pN+) population as well. Retrospective analyses of archived tissue from multiple clinical trials have found the RS to be prognostic in both endocrine therapy (ET)-treated and chemotherapy-treated patients with pN+ disease. Distribution of RS results in pN+ patients have also been consistent with those of pN0 populations. Data from the SWOG 8814 trial and large population-based registries further support the prognostic and potential predictive value of the RS. Specifically, patients with 1 to 3 positive nodes and RS less than 18 derived negligible benefit from adjuvant chemotherapy in these studies. In the prospective West German Study Group PlanB and ADAPT trials, pN+ patients with RS less than 11 and RS ≤25, respectively, who were treated with ET alone experienced excellent outcomes. Finally, 5-year results of the RxPONDER clinical trial randomizing patients with 1 to 3 positive nodes and RS ≤25 to ET alone vs ET plus chemotherapy confirmed an absence of chemotherapy benefit in postmenopausal patients. Clinical practice guidelines support use of the RS in the pN+, ER+/HER2– population, and many institutions have adopted the RS to guide clinical decision-making, resulting in a net reduction of adjuvant chemotherapy use. This review highlights the existing data supporting the prognostic and predictive ability of the RS in pN+ disease, current practice patterns related to RS use in this population, and emerging applications.
Oncology (Williston Park). 2021;35(2):77-84.
DOI: 10.46883/ONC.2021.3502.0077
Introduction
Insights from gene expression profiling have vastly expanded our understanding of distinct molecular subtypes of breast cancer, and of tumor biology’s influence on response to therapy and outcomes. Efforts to develop genomic assays for clinical use have resulted in a new era of molecularly tailored breast cancer management. The Oncotype DX Breast Recurrence Score® (RS) analyzes gene expression using a reverse transcriptase-polymerase chain reaction method. After evaluating 250 candidate genes in archived tissue from node-negative (pN0) estrogen receptor–positive (ER+) patients, a panel of 21 genes (16 cancer-related genes and 5 reference genes) was selected.1-3 A proprietary algorithm produces a recurrence score of 0 to 100, with a higher score correlating to an increased risk of distant recurrence (DR). Substantial efforts have focused on demonstrating the clinical utility of this tool, through both its prognostic and predictive value. For review, a prognostic tool provides information about the likelihood of a clinical outcome. A predictive tool provides information about the effect of an exposure (for example, a medical treatment) on a particular individual. To definitively demonstrate predictive value of the RS, a comparison is needed between patients randomized to either receive or not receive a treatment of interest, within RS categories.
The prognostic value of the RS and its ability to predict adjuvant chemotherapy benefit is well established in pN0 ER+ disease.4-6 The TAILORx study assigned patients with RS less than 11 to endocrine therapy (ET) alone and those with RS greater than 25 to ET plus chemotherapy; it randomized those with RS 11 to25 to ET with or without chemotherapy. Excellent outcomes with ET alone were demonstrated in patients with RS less than 11, with 96.8% free from DR at 9 years. Further, among patients with RS 11-25, ET alone was noninferior to ET plus chemotherapy overall, with a difference of less than 1% in 9-year DR rates between the 2 groups. In an exploratory analysis, a small reduction in DR was seen with ET plus chemotherapy in women 50 years or younger with RS 16-20 (1.6% absolute difference) and RS 21-25 (6.5% absolute difference). However, this may be explained by chemotherapy-induced ovarian function suppression, and the clinical relevance of this finding remains controversial.6
The RS has now been incorporated into the American Joint Committee on Cancer (AJCC) 8th edition staging system, such that all T1-2 N0 disease with RS less than 11 is classified as Prognostic Stage IA.7 The role of the RS for adjuvant systemic therapy decision-making in pN0 ER+ disease is supported in multiple clinical practice guidelines.8-10 In the TAILORx trial, 16% and 67% of patients had RS less than 11 and RS 11-25, respectively6; omitting chemotherapy in the majority of these patients has had substantive clinical impact. There are now retrospective and prospective data using the RS in node-positive (pN+) disease as well, including 5-year results from the RxPONDER clinical trial. In this study, patients with 1 to 3 positive nodes and RS ≤25 were randomized to receive ET with or without chemotherapy,11 providing level I evidence to support the clinical utility of the RS in pN+ disease. Here, we will review this literature in detail, along with published institutional experiences regarding the use of RS in pN+ disease and emerging applications for the RS beyond adjuvant chemotherapy decision-making.
Prognostic and Predictive Value of the RS: Retrospective Analyses of Clinical Trials
Studies supporting the prognostic and predictive ability of the RS are presented in Table 1 and Table 2, respectively. The first retrospective analysis of a prospective phase 3 clinical trial evaluating the RS in pN+ patients was performed in patients from the SWOG 8814 trial. This study evaluated tamoxifen alone vs chemotherapy (cyclophosphamide, doxorubicin, and fluorouracil) with concurrent or subsequent tamoxifen in postmenopausal pN+ patients. For a preplanned translational study, the RS was performed on banked tumor specimens. The prognostic ability of the RS was demonstrated in 148 patients from the tamoxifen-only arm, 64% and 36% of whom had 1 to 3 and 4 or more positive nodes, respectively. Ten-year disease-free survival (DFS) adjusted for the number of positive nodes was 60% in those with RS less than 18, 49% with RS 18-30, and 43% with RS of 31 or more (P = .017).12
TABLE 1. Summary of the studies demonstrating the prognostic value of the RS in pN+ patients
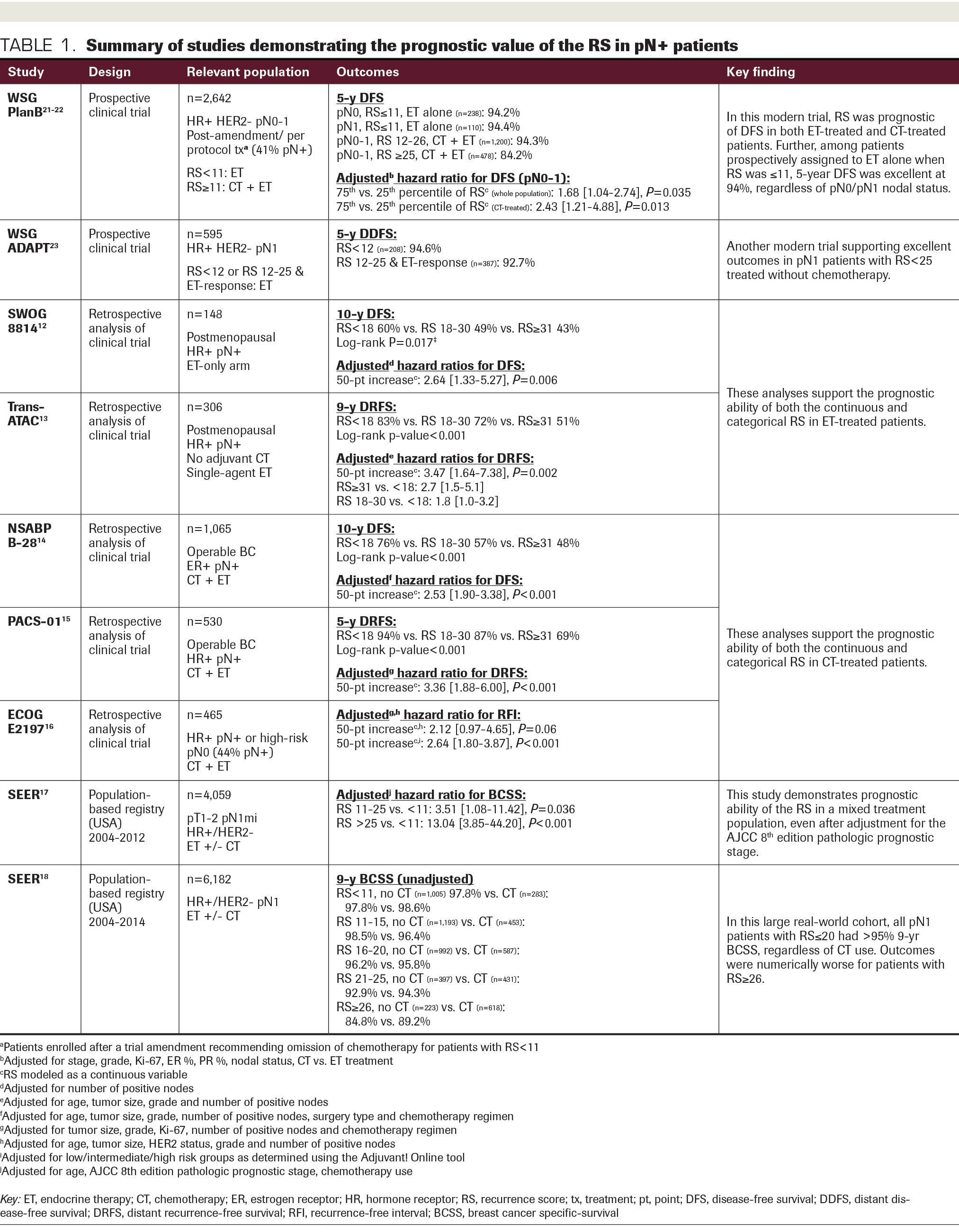
To evaluate the predictive value of the RS in SWOG 8814, outcomes were compared between patients in the tamoxifen-only arm (n = 148) and those who received chemotherapy followed by tamoxifen (n = 219). Among patients with RS less than 18 or RS 18-30, there was no DFS benefit with chemotherapy (RS <18: HR, 1.02; 95% CI, 0.54-1.93; P = .97; RS 18-30: HR, 0.72; 95% CI, 0.39-1.31; P = .48), whereas a significant benefit was observed in those with RS of 31 or more (HR, 0.59; 95% CI, 0.35-1.01; P = .033). Results were similar for breast cancer–specific survival (BCSS) and overall survival (OS). Adjusting for number of positive nodes, there was a significant interaction between the continuous RS and chemotherapy effect on DFS (P = .053), particularly in the first 5 years (P = .029).12 While these data are compelling, the sample size was small and the study may have been underpowered. In addition, the parent trial was conducted more than 25 years ago and is thus not reflective of modern therapy.
Multiple additional “retrospective-prospective” studies lend further support to the prognostic ability of the RS in both ET-treated and chemotherapy-treated pN+ patients. The TransATAC trial randomized postmenopausal patients to adjuvant anastrozole, tamoxifen, or a combination of the 2. Among 306 pN+ patients from the single-agent arms treated without chemotherapy, both the continuous and categorical RS were independent predictors of DR and OS on adjusted analysis.13 The NSABP B-28 trial randomized pN+ patients to doxorubicin and cyclophosphamide chemotherapy with or without paclitaxel. RS was performed retrospectively in 1065 patients; 10-year DFS was 75.8% for RS less than 18, 57.0% for RS 18-30, and 48.0% for RS of 31 or more (P < .001). RS was also correlated with distant recurrence-free survival (DRFS), OS, and BCSS, and the association of the continuous RS with each outcome remained significant on adjusted analyses (HRs for 50-point increase, 2.42-3.38; all P < .001).14
Among 530 pN+ patients from the PACS-01 trial randomized to fluorouracil, epirubicin, and cyclophosphamide chemotherapy with or without docetaxel, the continuous RS was again strongly associated with DR, DFS, and OS on multivariable analysis (all P < .001).15 Finally, the ECOG E2197 trial included pN+ and high-risk pN0 patients, randomized to doxorubicin with either cyclophosphamide or docetaxel. Among 465 patients, 44% had 1 to 3 positive lymph nodes. The RS outperformed a modified version of the Adjuvant! Online tool, which uses traditional clinicopathologic risk factors, in predicting 5-year risk of recurrence. The prognostic effect of the RS remained for all levels of Adjuvant! Online risk assessment.16
TABLE 2. Summary of studies evaluating oncologic outcomes in pN+ patients stratified by use of chemotherapy and RS.
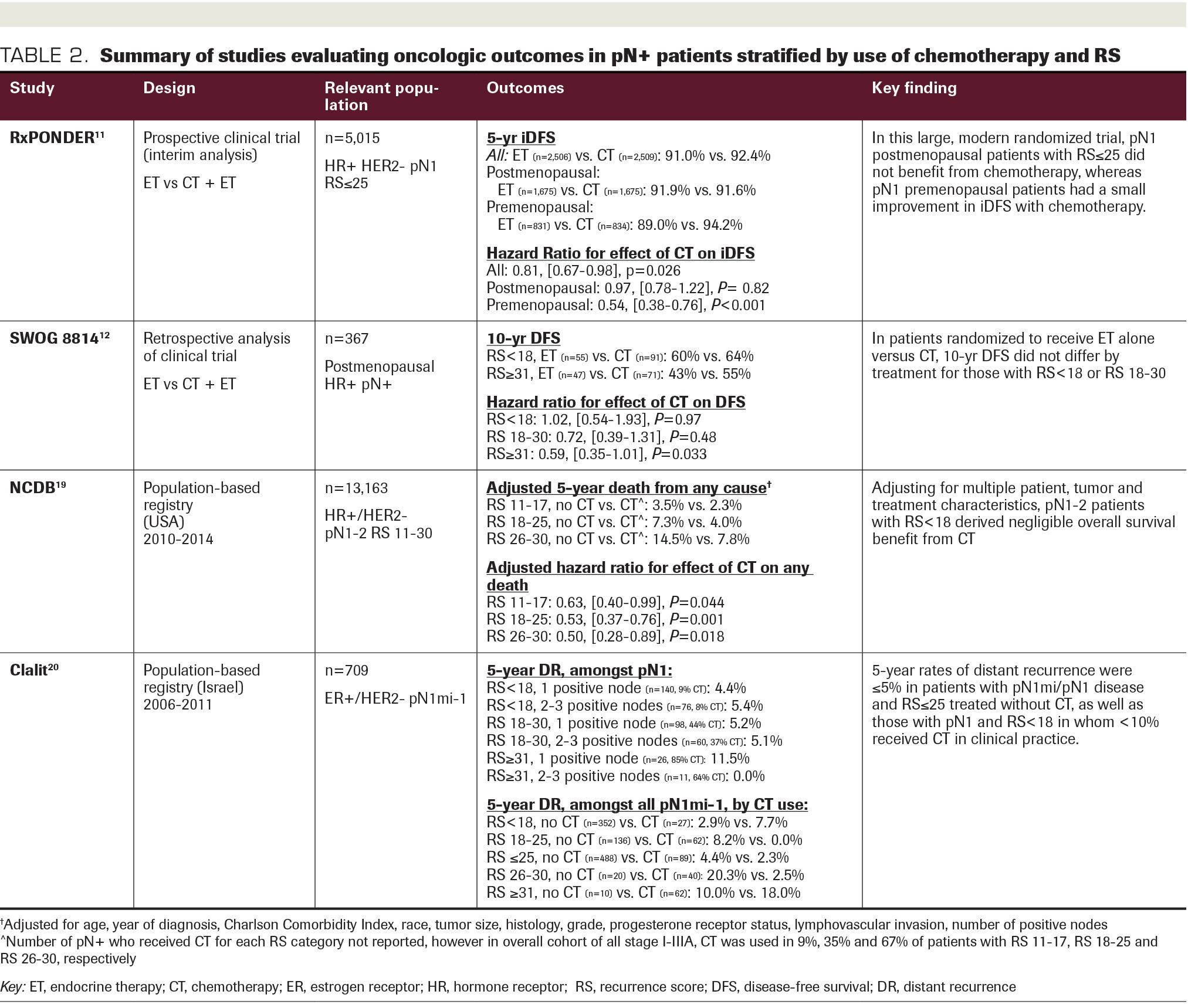
The distribution of RS results was very consistent in the SWOG 8814, NSABP B-28, and PACS-01 pN+ populations; less than 18 in 36%-40%, 18-30 in 28%-34%, and 31or greater in
30%-32%.12,14-15 TransATAC patients had a higher proportion of RS less than 18 (52%) and a lower proportion of RS of 31 or greater (17%),13 although this analysis excluded patients treated with chemotherapy, which likely explains this finding. In historical studies validating the prognostic ability of the RS in pN0 patients, the distribution of RS results was less than 18 in 51%-54%, 18-30 in 21%-22%, and 31 or greater in 25%-27%.4-5 Thus, while the mean RS may be slightly higher in pN+ patients, the distribution is not substantially different than in the pN0 population.
The percentage of patients evaluated in the above trials with 1 to 3 positive nodes vs 4 or more positive nodes ranged from 60% to 79%.12-15 In NSABP B-28, the distribution of RS results was similar between patients with 1 to 3 vs 4 or more positive nodes.14 Further, the RS remained prognostic in both those with 1 to 3 and 4 or more positive nodes in the TransATAC, NSABP B-28, and PACS-01 studies.13-15 In the PACS-01 study, patients with 4 or more nodes and an RS less than 18 had a 5-year DR rate of less than 10%, which was similar to those with 1 to 3 nodes and RS less than 18 or 18-30, and lower than those with 1 to 3 positive nodes and RS of 31 or greater.15 Similarly, in NSABP B-28, 10-year DFS and BCSS in those with 4 or more nodes and RS less than 18 were similar or greater than in patients with 1 to 3 positive nodes and RS 18-30 or 31 or greater.14 Overall, these findings suggest that the RS may have a role even in patients with 4 or more nodes, a population that is not included in the RxPONDER trial.
Prognostic and Predictive Value of the RS: Population-Based Studies
Population-based series have further confirmed the prognostic ability of the RS in larger, “real-world” samples of pN+ patients. These studies provide support for the predictive value of RS as well, although they are limited by the fact that chemotherapy use is not randomized but selected. Wang et al evaluated outcomes by TAILORx RS cut points in 4059 pT1-2 pN1 patients from the Surveillance, Epidemiology and End Results (SEER) database. Patients with RS 11-25 and RS greater than 25 vs RS less than 11 experienced inferior BCSS and OS, independent of the AJCC 8th edition pathologic prognostic stage.17 In a larger, more recent SEER study of greater than 6000 pN1 patients, unadjusted rates of 9-year breast cancer–specific mortality were less than 5% for RS up to 20, regardless of chemotherapy use. Notably, in those treated without chemotherapy, the differences in breast cancer-specific mortality between pN0 and pN1 patients were less than 1% for RS 0-15 and less than 2% for RS 16-20.18
An analysis of the National Cancer Database (NCDB) included 13,163 pN+ patients with an intermediate RS, defined as 11-30, and stratified into groupings of 11-17, 18-25, and 26-30. On multivariable analysis, 5-year risk of mortality by chemotherapy use statistically differed for all RS groupings; however, in those with RS 11-17, the absolute benefit was only 1.3% (HR, 0.63; 95% CI, 0.40-0.99; P = .044). With RS 18-25 and 26-30, the use of chemotherapy was associated with larger gains in OS (3.3% and 6.7% absolute benefit, respectively). When the continuous RS score was modeled, no benefit from chemotherapy was seen for an RS less than 14.19 Neither the SEER database nor NCDB capture DFS, a limitation of these series as patients with ER+/HER2– disease may survive for years with recurrent or metastatic disease.
Finally, in an analysis of 709 patients with micrometastases (pN1mi) or pN1 disease from the Israeli Clalit Health Services registry, adjuvant chemotherapy was used in 9% with RS less than 18, 41% with RS 18-30, and 78% with RS of 31 or greater in the pN1 subset. Five-year DR rates were 5% or less for those with RS less than 18 or RS 18-30, regardless of having 1 vs 2 to 3 positive nodes. Among all patients (pN1mi-pN1) treated without chemotherapy, 5-year DR rates were 2.9% for RS less than 18. The RxPONDER cut point of 25 was also evaluated; chemotherapy was used in only 15% of those with RS of 25or less, in whom the 5-year DR rate was 4.4%, vs 2.3% in those treated without chemotherapy (P = .521).20
Prognostic and Predictive Value of the RS: Prospective Validation and Summary
The West German Study Group (WSG) PlanB trial was the first to prospectively validate the prognostic ability of the RS in pN+ disease, as well as the ability of the RS to identify a subset of pN+ patients with excellent outcomes in the absence of chemotherapy. Initially designed to compare chemotherapy regimens with or without anthracyclines, this study included 2449 pN+ and high-risk pN0 patients. An early amendment recommended omission of chemotherapy for all patients with RS of 11 or less, regardless of nodal status. In both the overall population and the chemotherapy-treated subgroup (with RS >11), the continuous RS was an independent predictor of DFS.21 Further, as a result of the early amendment, chemotherapy was omitted in 86% of patients with RS of 11 or less. Among those who received ET alone, 5-year DFS was excellent at 94.2%, and it did not differ significantly between pN1 and pN0 patients (94.2% and 94.4%, respectively).22
Findings from 2 important clinical trials using the RS in pN+ disease were reported at the San Antonio Breast Cancer Symposium in December 2020. In the WSG ADAPT trial, patients who were considered candidates for adjuvant chemotherapy by conventional criteria received a short (3-week) course of preoperative ET, followed by surgery. Those with pN0-1 disease and RS less than 12, or RS 12-25 plus evidence of response to ET (defined by Ki67 expression ≤10% on surgical pathology) were treated with ET alone. In the subgroup of 595 patients with pN1 disease, 5-year distant DFS was excellent, and it did not significantly differ between those with RS 0-11 vs RS 12-25 (94.6% vs 92.7%, respectively).23
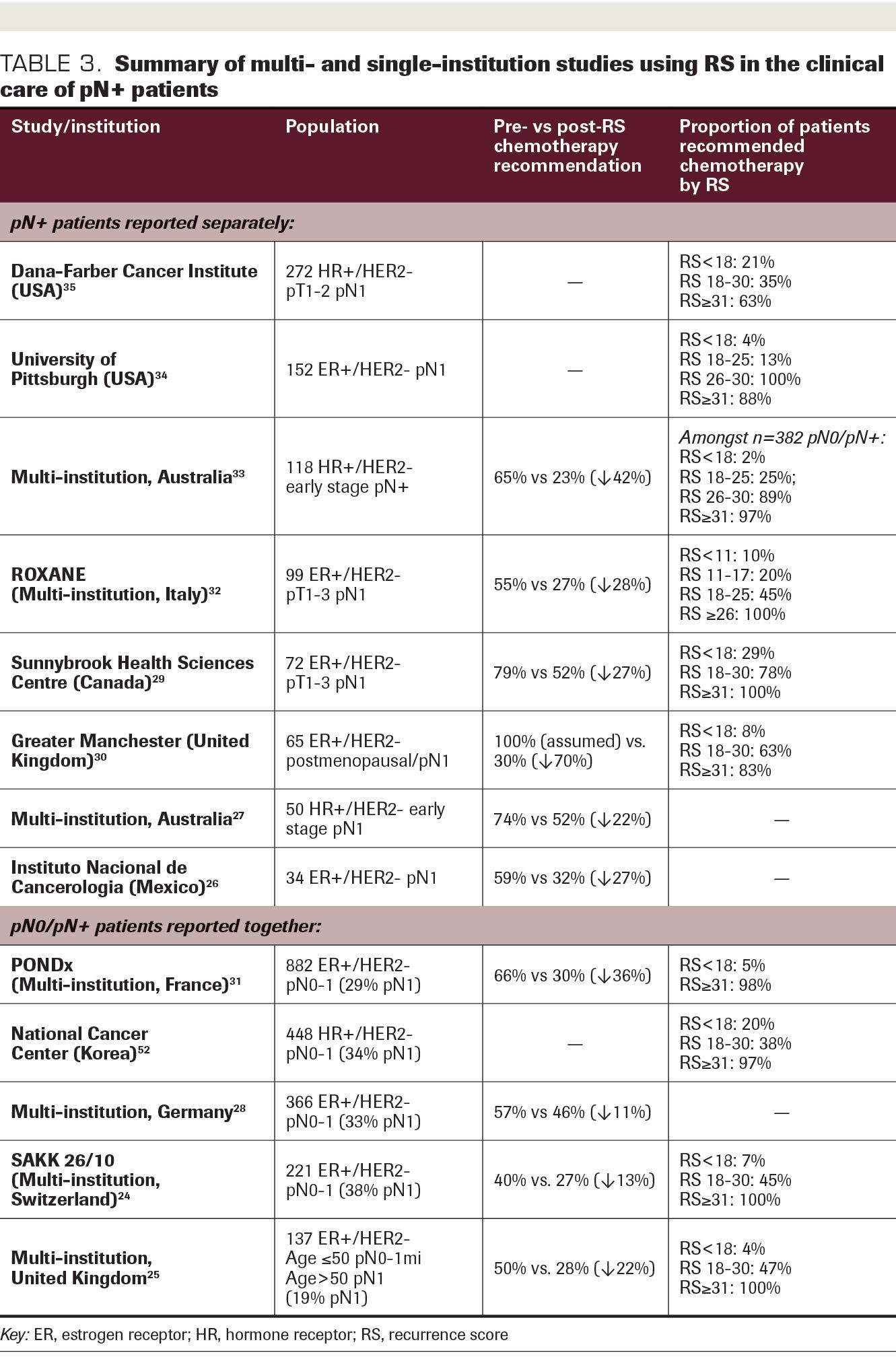
TABLE 3. Summary of multi- and single- institution studies using RS in the clinical care of pN+ patients.
Finally, highly anticipated 5-year results were presented from RxPONDER, the first trial to randomize chemotherapy use in 5015 patients with 1 to 3 positive nodes and RS of 25 or less. Chemotherapy benefit did not differ by the continuous RS (P = .30) but did differ by menopausal status (P = .008). In postmenopausal patients, there was no difference in 5-year invasive DFS (iDFS) or OS between treatment arms. In premenopausal patients, 5-year iDFS was significantly improved in the ET plus chemotherapy group, with an absolute difference in iDFS of 5.2%. A small (1.3%) but statistically significant OS benefit was also seen in premenopausal patients with ET plus chemotherapy.11 Similar to the age-based differences in chemotherapy benefit observed in the TAILORx trial, some or all of the benefit from chemotherapy in premenopausal women could be a result of the indirect effect of chemotherapy on ovarian function. Two findings in subgroup analyses of premenopausal women suggest that chemotherapy-induced ovarian suppression is playing a role. First, the benefits of chemotherapy were as great or greater in those with a lower RS category (RS 0-13, HR, 0.45; vs RS 14-25, HR, 0.56), which would be consistent with an endocrine effect. Second, there was an absence of significant chemotherapy benefit in premenopausal women 50 years or greater (≥50 years, HR, 0.84; vs <50 years, HR, 0.43-0.44), who would be close to natural menopause and less likely to gain the benefit of chemotherapy-induced menopause.
In summary, the RS has consistently shown a strong association with oncologic outcomes in both ET-treated and chemotherapy-treated patients, independent of traditional risk factors such as age, tumor size, grade, and number of positive nodes. Further, data from SWOG 8814 and multiple population-based registries strongly support that pN1 patients with RS less than 18 have excellent oncologic outcomes in the absence of chemotherapy. The WSG PlanB and ADAPT trials provide prospective validation of excellent outcomes with ET alone in patients with RS 11 or less and RS of 25 or less, respectively. Finally, findings from RxPONDER demonstrate that ET alone provides equivalent outcomes to ET plus chemotherapy in postmenopausal pN1 patients with RS of 25 or less. Though small benefits from chemotherapy were observed in premenopausal patients in RxPONDER, chemotherapy-induced ovarian function suppression may be driving this finding. As a result of this substantial body of evidence, the National Comprehensive Cancer Network guidelines support the 21-gene RS to assist in adjuvant chemotherapy decision-making for patients with hormone receptor–positive/HER2– pN1 disease.8
Multi- and Single-Institution Experience Using RS in
pN+ Patients
Numerous institutions across North America, Europe, Asia, and Australia have reported on their experience incorporating the use of the RS into adjuvant chemotherapy decision-making in ER+/HER2– disease (Table 3). Changes in treatment recommendations based on RS results have ranged from 11% to 42%.24-33 While many series include both pN0 and pN1 patients, recommendation changes are often greatest in the pN1 group.24,26-28,32 A multicenter Australian study of 382 patients included 118 (32%) who were pN+; of these, chemotherapy was recommended in 65% pre-RS versus 23% post RS. In the overall cohort, 98% with RS less than 18 and 78% with RS 18-30 were ultimately treated with ET alone, while 97% with RS 31 or greater received chemotherapy.33 The prospective ROXANE study included 251 pN0-1 patients from 9 centers in Italy, of whom 99 (39%) were pN1. Of these, 55% were recommended chemotherapy pre-RS versus 27% post RS; this reduction in chemotherapy use was observed exclusively in patients with RS of 17 or less.32 Similarly, in a prospective Canadian cohort of 72 pN1 patients, changes in treatment recommendation were most common for RS less than 18. In this group, 76% were recommended chemotherapy pre-RS, nearly two-thirds of whom were subsequently recommended ET alone post RS.29
The University of Pittsburgh Cancer Center has developed and implemented a clinical pathway for patients with ER+/HER2– pN0-1 disease, in which those with RS less than 18 are recommended ET alone, those with RS greater than 30 are recommended chemotherapy, and those with RS 18-30 are left to the discretion of the treating oncologist. In their experience, use of RS was high in eligible patients (93%). Among patients with 1 to 3 positive nodes, there was strong adherence to the pathway; chemotherapy was used in 4% of those with RS less than 18, 25% with RS 18-30, and 88% with an RS of 31 or greater.34
At the Dana-Farber Cancer Institute, since 2016, we have performed “reflex” RS testing for patients aged 65 years or less with hormone receptor–positive/HER2– pT1-2 pN1 disease; RS can also be ordered outside of these criteria at the oncologists’ discretion. In 347 women with pN1 disease treated with upfront surgery between 2016 and 2019, 272 (78%) had RS testing. Chemotherapy was used in 21.0% with RS less than 18, 34.8% with RS 18-30, and 62.5% for RS 31 or greater (P = .01). Furthermore, RS less than 18 was a significant predictor of chemotherapy use on adjusted analysis (odds ratio, 0.47; 95% CI, 0.24-0.92; P = .028). While follow-up was limited in duration (median, 27.0 months), our study is among the few institutional experiences to report outcome data. In the overall cohort, we observed no difference in 3-year recurrence-free survival in those treated with or without chemotherapy (97.7% vs 97.5%; P = .97), nor were there differences in 3-year OS (98.5% vs 100%; P = .19).35
Special Populations
Consideration for the available evidence in underrepresented populations, including young patients, elderly patients, and patients of ethnic minorities, is warranted. While the SWOG 8814 and TransATAC studies evaluated only postmenopausal patients, 48% and 53% of subjects in the NSABP B-28 and PACS 01 analyses were aged less than 50 years,14-15 and 24% in the ECOG E2197 study were aged 45 years or less.16 In the NCDB and Clalit registry studies, women less than 50 years accounted for 15% to 24% of the study populations.19-20 In the prospective trials, median age in the overall hormone receptor–positive cohort of WSG PlanB was 56 years.22 Approximately one-third of the patients in the RxPONDER trial were premenopausal, although a specific age breakdown has not been reported.11
The prognostic impact of the RS has been evaluated in the Young Women’s Breast Cancer Study, a multicenter prospective cohort of women aged 40 years or less at diagnosis. Among 163 pN1 patients, RS distribution was similar to nonyoung cohorts: 33% had RS less than 18, 42% had RS 18-30, and 25% had RS 31 or greater. Chemotherapy use was high overall: 83%, 97%, and 97%, respectively. The categorical RS was prognostic; 6-year DRFS was similar for RS less than 18 and RS 18-30 (85.9% and 87.3%, respectively), but markedly worse (62.8%) for RS 31 or greater (P = .004). Using TAILORx categories, 6-year DRFS for RS 11-25 was similar to the RS less than 18 and RS 18-30 categories (85.2%), but substantially lower for RS greater than 25 (71.3%).36
The utility of RS in older women was evaluated in a SEER study of pN0 and pN+ patients that compared patients aged more than 70 years with those 18 to 69 years. In the older cohort, 18% were pN+; the distribution of RS results was similar between age groups. On adjusted analysis, RS greater than 25 was associated with inferior OS compared with RS less than 11 in both age groups, although the magnitude of effect was lower in the older group (>70 years: HR, 1.47, P = .003; 18-69 years: HR, 2.35, P < .001). Among patients with RS greater than 25, chemotherapy was used in 73% and 52% of those aged 18 to 69 years and greater than 70 years, respectively. Use of chemotherapy was significantly associated with improved adjusted OS and BCSS in the younger cohort, but not in patients aged more than 70 years.37
Few studies have compared the clinical value of the RS across ethnic groups. A recent NCDB analysis of pN0 and pN+ patients identified small but statistically significant differences in the prognostic performance of the RS across racial/ethnic groups (P <.001). The c-index was used to compare the predictive ability per 10-unit RS increase, where a value of 1 indicates perfect prediction and a value of 0.5 indicates no better predictive ability than random chance. The c-index was highest for patients who were non-Hispanic White (0.581) and Asian/Pacific Islander (0.586), and lowest for those who were African American (0.535) and Hispanic (0.542).38 Further study is warranted in minority populations, who are also underrepresented in clinical trials.
Other Emerging Applications
There is increasing interest in incorporating the 21-gene RS into other elements of clinical decision-making beyond those choices related to adjuvant chemotherapy. In pN0 patients, gene expression profiles can prognosticate likelihood of locoregional recurrence (LRR)39,40; this has now been shown in pN+ disease as well. Among 1065 pN+ patients from NSABP B-28, 43% were treated with breast-conserving surgery (BCS) and 57% with mastectomy. The 10-year LRR rate was 3.3% for those with RS less than 18, 7.2% with RS 18-30, and 12.2% with RS of 31 or greater
(P < .001). On adjusted analysis, the continuous RS remained a significant predictor of LRR; for any 50-point increase in the RS result, the risk of LRR more than doubled (HR for 50-point increase, 2.59; 95% CI, 1.28-5.26; P = .008).41
Among 316 pN+ patients treated with BCS and radiation or mastectomy without radiation in SWOG 8814, 10-year LRR rates were 9.7% for RS less than 18 versus 16.5% for RS ≥18 (P = .02), and an RS 18 or greater remained significantly associated with LRR after adjustment for surgery type, number of positive nodes, and use of chemotherapy. In the mastectomy subgroup, 10-year LRR was particularly low (1.5%) in those with 1 to 3 positive nodes and RS less than 18. In patients with 4 or more positive nodes, 10-year LRR was similar regardless of RS category (RS <18, 25.9% vs RS ≥18, 27.0%; P = .27).42 Together, these findings suggest a potential role for tailoring use of radiation therapy based on RS results. The ongoing TAILOR RT trial will be the first randomized control trial evaluating this concept, with a recurrence-free interval primary end point. Patients with ER+/HER2– disease, 1 to 3 positive nodes, and RS less than 18 are eligible; those treated with BCS are randomized to whole breast radiation with or without regional nodal radiation, and those treated with mastectomy are randomized to no radiation or chest wall/regional nodal radiation.43
Another potential application for the 21-gene RS in pN+ patients is to guide neoadjuvant systemic therapy decisions. The feasibility and reliability of performing the RS assay on core biopsy specimens has been demonstrated in greater than 100,000 samples.44 Unlike other biologic subtypes, hormone receptor–positive patients are eligible for either neoadjuvant chemotherapy (NAC) or neoadjuvant endocrine therapy (NET). Rates of pathologic complete response (pCR) to NAC are low in this population,45 and even lower in patients with low or intermediate RS.46-48 Further, a meta-analysis of nearly 3500 patients demonstrated that NET is associated with equivalent response rates and rates of BCS, but with lower toxicity compared with NAC.49 Therefore, using the RS to identify patients who may not require chemotherapy as part of their treatment could help select those most suited to an NET approach. A small but prospective, multicenter study evaluated the feasibility of selecting NAC vs NET based on the RS in 64 pN0-1 patients. NET was recommended for RS less than 11 and NAC for RS greater than 25, and those with RS 11-25 were randomized to either regimen; compliance with assigned treatment was high in the randomized arm (85%), supporting feasibility of this strategy.50 We anticipate that oncologists will increasingly incorporate the RS into neoadjuvant treatment recommendations in patients with hormone receptor–
positive disease.
Conclusions
Substantial existing data support the clinical utility of the RS in patients with hormone receptor–positive/HER2– pN+ breast cancer. In particular, those with 1 to 3 positive nodes and RS less than 18 have excellent outcomes and appear to derive negligible benefit from adjuvant chemotherapy in retrospective analyses of clinical trials and large population-based series. Prospective validation of these findings is provided by the excellent outcomes observed with ET alone in those with RS less than 11 and RS of 25 or less in the WSG’s PlanB and ADAPT trials, as well as an absence of chemotherapy benefit in postmenopausal women with RS of 25 or less in RxPONDER. Premenopausal patients have been underrepresented in many studies; while RxPONDER demonstrated a small benefit to chemotherapy in premenopausal patients with RS of 25 or less, future analyses are needed to determine the attributable effect of chemotherapy-induced ovarian function suppression in this population. Based on existing retrospective data, we may be able to cautiously extrapolate the findings in postmenopausal women in RxPONDER to patients with 4 or more positive nodes as well. It is unlikely that there will ever be a prospective randomized trial of ET vs ET plus chemotherapy in this group of women with multinode-positive breast cancer.
While the focus of this review was the 21-gene recurrence score, the MINDACT trial now provides prospective evidence for the use of another genomic assay in adjuvant chemotherapy decision-making in pN+ patients. In this phase 3 study, pN0-1 patients with high clinical-risk but low genomic-risk disease (determined by the MammaPrint® 70-gene signature test) were randomized to ET alone vs ET plus chemotherapy. In a preplanned subset analysis of 738 patients with pN1 disease, DRFS was 96.3% with chemotherapy vs 95.6% with ET alone. 51 This lends further support for the ability of genomic assays to select patients who do not benefit from adjuvant chemotherapy. We have highlighted that many institutions have already adopted the RS assay in clinical decision-making for adjuvant chemotherapy in the pN1 population, with a resultant net reduction in chemotherapy use. Emerging applications for the RS in clinical care include decision-making related to radiation therapy as well as selection of neoadjuvant systemic therapy.
References
- Cobleigh MA, Tabesh B, Bitterman P, et al. Tumor gene expression and prognosis in breast cancer patients with 10 or more positive lymph nodes. Clin Cancer Res. 2005;11(24 Pt 1):8623-8631. doi:10.1158/1078-0432.CCR-05-0735
- Esteban J, Baker J, Cronin M, et al. Tumor gene expression and prognosis in breast cancer: multi-gene RT-PCR assay of paraffin-embedded tissue. Proc Am Soc Clin Oncol. 2003;22:850.
- Paik S, Shak S, Tang G, et al. Multi-gene RT-PCR assay for predicting recurrence in node negative breast cancer patients: NSABP studies B-20 and B-14. Breast Cancer Res Treat. 2003;82:abstr A16.
- Paik S, Shak S, Tang G, et al. A multigene assay to predict recurrence of tamoxifen treated node-negative breast cancer. N Engl J Med. 2004;351(27):2817-2826. doi:10.1056/NEJMoa041588
- Paik S, Tang G, Shak S, et al. Gene expression and benefit of chemotherapy in women with node-negative, estrogen receptor-positive breast cancer. J Clin Oncol. 2006;24(23):3726-3734. doi:10.1200/JCO.2005.04.7985
- Sparano JA, Gray RJ, Makower DF, et al. Adjuvant chemotherapy guided by a 21-gene expression assay in breast cancer. N Engl J Med. 2018;379(2):111-121. doi:10.1056/NEJMoa1804710
- Guiliano AE, Connolly JL, Edge SB, et al. Breast cancer – major changes in the American Joint Committee on Cancer eighth edition cancer staging manual. CA Cancer J Clin. 2017;67(4):290-303. doi:10.3322/caac.21393
- NCCN. Clinical Practice Guidelines in Oncology. Breast cancer, version 6.2020. Accessed September 27, 2020. https://www.nccn.org/professionals/physician_gls/pdf/breast.pdf
- Andre F, Ismaila N, Henry NL, et al. Use of biomarkers to guide decisions on adjuvant systemic therapy for women with early-stage invasive breast cancer: ASCO Clinical Practice Guideline Update – integration of results from TAILORx. J Clin Oncol. 2019;37(22):1956-1964. doi:10.1200/JCO.19.00945
- Cardoso F, Kyriakides S, Ohno S, et al; ESMO Guidelines Committee. Early breast cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2019;30(8):1194-1220. doi:10.1093/annonc/mdz173
- Kalinsky K, Barlow WE, Meric-Bernstam F, et al. First results from a phase III randomized clinical trial of standard adjuvant endocrine therapy (ET) +/– chemotherapy (CT) in patients (pts) with 1-3 positive nodes, hormone receptor-positive (HR+) and HER2-negative (HER2–) breast cancer (BC) with recurrence score (RS) ≤ 25: SWOG S1007 (RxPonder). Presented at: 2020 San Antonio Breast Cancer Symposium; December 8-11, 2020; virtual. Abstract GS3-00. https://www.abstractsonline.com/pp8/#!/9223/presentations/rxponder/1
- Albain KS, Barlow WE, Shak S, et al. Prognostic and predictive value of the 21-gene recurrence score assay in postmenopausal women with node-positive, estrogen-receptor-positive breast cancer on chemotherapy: a retrospective analysis of a randomised trial. Lancet Oncol. 2010;11(1):55-65. doi:10.1016/S1470-2045(09)70314-6
- Dowsett M, Cuzick J, Wale C, et al. Prediction of risk of distant recurrence using the 21-gene recurrence score in node-negative and node-positive postmenopausal patients with breast cancer treated with anastrozole or tamoxifen: a TransATAC study. J Clin Oncol. 2010;28(11):1829-1834. doi:10.1200/JCO.2009.24.4798
- Mamounas EP, Tang G, Paik S, et al. 21-Gene Recurrence Score for prognosis and prediction of taxane benefit after adjuvant chemotherapy plus endocrine therapy: results from NSABP B-28/NRG Oncology. Breast Cancer Res Treat. 2018;168(1):69-77. doi:10.1007/s10549-017-4550-8
- Penault-Llorca F, Filleron T, Asselain B, et al. The 21-gene Recurrence Score assay predicts distant recurrence in lymph node-positive, hormone receptor-positive, breast cancer patients treated with adjuvant sequential epirubicin- and docetaxel-based or epirubicin-based chemotherapy (PACS-01 trial). BMC Cancer. 2018;18(1):526. doi:10.1186/s12885-018-4331-8
- Goldstein LJ, Gray R, Badve S, et al. Prognostic utility of the 21-gene assay in hormone-receptor-positive operable breast cancer compared with classical clinicopathologic features. J Clin Oncol. 2008;26(25):4063-4071. doi:10.1200/JCO.2007.14.4501
- Wang M, Wu K, Zhang P, Zhang M, Ding A, Chen H. The prognostic significance of the Oncotype DX recurrence score in T1-2 N1 M0 estrogen receptor-positive HER2-negative breast cancer based on the prognostic stage in the updated AJCC 8th edition. Ann Surg Oncol. 2019;26(5):1227-1235. doi:10.1245/s10434-018-7068-3
- Hortobagyi GN, Shak S, Sledge GW Jr, et al. Breast cancer-specific mortality (BCSM) in patients (pts) with node-negative (N0) and node-positive (N+) breast cancer (BC) guided by the 21-gene assay: a SEER-genomic population-based study. Cancer Res. 2019;79(4 Suppl):abstr P3-11-02. doi:10.1158/1538-7445.SABCS18-P3-11-02
- Ibraheem AF, Press DJ, Olopade OI, Huo D. Community clinical practice patterns and mortality in patients with intermediate oncotype DX recurrence scores: who benefits from chemotherapy? Cancer. 2019;125(2):213-222. doi:10.1002/cncr.31818
- Stemmer SM, Steiner M, Rizel S, et al. Clinical outcomes in ER+ HER2– node-positive breast cancer patients who were treated according to the Recurrence Score results: evidence from a large prospectively designed registry. NPJ Breast Cancer. 2017;3:32. doi:10.1038/s41523-017-0033-7
- Gluz O, Nitz UA, Christgen M, et al. West German Study Group Phase III PlanB Trial: first prospective outcome data for the 21-gene recurrence score assay and concordance of prognostic markers by central and local pathology assessment. J Clin Oncol. 2016:34(20):2341-2349. doi:10.1200/JCO.2015.63.5383
- Nitz U, Gluz O, Christgen M, et al. Reducing chemotherapy use in clinically high-risk, genomically low-risk pN0 and pN1 early breast cancer patients: five-year data from the prospective, randomised phase 3 West German Study Group (WSG) PlanB trial. Breast Cancer Res Treat. 2017;165(3):573-583. doi:10.1007/s10549-017-4358-6
- Harbeck N, Gluz O, Kuemmel S, et al; West German Study Group. Endocrine therapy alone in patients with intermediate or high-risk luminal early breast cancer (0-3 lymph nodes), Recurrence Score <26 and Ki67 response after preoperative endocrine therapy: primary outcome results from the WSG-ADAPT HR+/HER2– trial. Presented at: 2020 San Antonio Breast Cancer Symposium; December 8-11, 2020; virtual. Abstract GS3-00. https://www.abstractsonline.com/pp8/#!/9223/presentation/682
- Pestalozzi BC, Tausch C, Dedes KJ, et al; Swiss Group for Clinical Cancer Research (SAKK). Adjuvant treatment recommendations for patients with ER-positive/HER2-negative early breast cancer by Swiss tumor boards using the 21-gene recurrence score (SAKK 26/10). BMC Cancer. 2017;17(1):265. doi:10.1186/s12885-017-3261-1
- Kuchel A, Robinson T, Comins C, et al. The impact of the 21-gene assay on adjuvant treatment decisions in oestrogen receptor-positive early breast cancer: a prospective study. Br J Cancer. 2016;114(7):731-736. doi:10.1038/bjc.2016.48
- Bargallo JE, Lara F, Shaw-Dulin R, et al. A study of the impact of the 21-gene breast cancer assay on the use of adjuvant chemotherapy in women with breast cancer in a Mexican public hospital. J Surg Oncol. 2015;111(2):203-207. doi:10.1002/jso.23794
- De Boer RH, Baker C, Speakman D, Chao CY, Yoshizawa C, Mann GB. The impact of a genomic assay (Oncotype DX) on adjuvant treatment recommendations in early breast cancer. Med J Aust. 2013;199(3):205-208. doi:10.5694/mja12.11334
- Eiermann W, Rezai M, Kümmel S, et al. The 21-gene recurrence score assay impacts adjuvant therapy recommendations for ER-positive, node-negative and node-positive early breast cancer resulting in a risk-adapted change in chemotherapy use. Ann Oncol. 2013;24(3):618-624. doi:10.1093/annonc/mds512
- Torres S, Trudeau M, Gandhi S, et al. Prospective evaluation of the impact of the 21-gene recurrence score assay on adjuvant treatment decisions for women with node-positive breast cancer in Ontario, Canada. Oncologist. 2018;23(7):768-775. doi:10.1634/theoncologist.2017-0346
- Loncaster J, Armstrong A, Howell S, et al. Impact of Oncotype DX breast recurrence score testing on adjuvant chemotherapy use in early breast cancer: real world experience in Greater Manchester, UK. Eur J Surg Oncol. 2017;43(5):931-937. doi:10.1016/j.ejso.2016.12.010
- Curtit E, Vannetzel J-M, Darmon J-C, et al. Results of PONDx, a prospective multicenter study of the Oncotype DX breast cancer assay: real-life utilization and decision impact in French clinical practice. Breast. 2019;44:39-45. doi:10.1016/j.breast.2018.12.015
- Dieci MV, Guarneri V, Zustovich F, et al; Veneto Oncology Network. Impact of 21-gene breast cancer assay on treatment decision for patients with T1-T3, N0-N1, estrogen-receptor positive/human epidermal growth receptor 2-negative breast cancer: final results of the prospective multicenter ROXANE study. Oncologist. 2019;24(11):1424-1431. doi:10.1634/theoncologist.2019-0103
- Chin-Lenn L, De Boer RH, Segelov E, et al. The impact and indications for Oncotype DX on adjuvant treatment recommendations when third-party funding is unavailable. Asia Pac J Clin Oncol. 2018;14(6):410-416. doi:10.1111/ajco.13075
- Ellis PG, Brufsky AM, Beriwal S, et al. Pathways clinical decision support for appropriate use of key biomarkers. J Oncol Pract. 2016;12(6):e681-e687. doi:10.1200/JOP.2015.010546
- Losk K, Freedman RA, Laws A, et al. Oncotype DX testing in node-positive breast cancer strongly impacts chemotherapy use at a comprehensive cancer center. Published online September 16, 2020. Breast Cancer Res Treat. doi:10.1007/s10549-020-05931-9
- Poorvu PD, Gelber SI, Rosenberg SM, et al. Prognostic impact of the 21-gene recurrence score assay among young women with node-negative and node-positive ER-positive/HER2-negative breast cancer. J Clin Oncol. 2020;38(7):725-733. doi:10.1200/JCO.19.01959
- Kizy S, Altman AM, Marmor S, et al. 21-gene recurrence score testing in the older population with estrogen receptor-positive breast cancer. J Geriatr Oncol. 2019;10(2):322-329. doi:10.1016/j.jgo.2018.07.006
- Ibraheem A, Olopade OI, Huo D. Propensity score analysis of the prognostic value of genomic assays for breast cancer in diverse populations using the National Cancer Data Base. Cancer. 2020;126(17):4013-4022. doi:10.1002/cncr.32956
- Mamounas EP, Tang G, Fisher B, et al. Association between the 21-gene recurrence score assay and risk of locoregional recurrence in node-negative, estrogen receptor-positive breast cancer: results from NSABP B-14 and NSABP B-20. J Clin Oncol. 2010;28(10):1677-1683. doi:10.1200/JCO.2009.23.7610
- Nuyten DSA, Kreike B, Hart AAM, et al. Predicting a local recurrence after breast-conserving therapy by gene expression profiling. BreastCancer Res. 2006;8(5):R62. doi:10.1186/bcr1614
- Mamounas EP, Liu Q, Paik S, et al. 21-gene recurrence score and locoregional recurrence in node-positive/ER-positive breast cancer treated with chemo-endocrine therapy. J Natl Cancer Inst. 2017;109(4):djw259. doi:10.1093/jnci/djw259
- Woodward WA, Barlow WE, Jagsi R, et al. Association between 21-gene assay recurrence score and locoregional recurrence rates in patients with node-positive breast cancer. JAMA Oncol. 2020;6(4):505-511. doi:10.1001/jamaoncol.2019.5559
- Regional radiotherapy in biomarker low risk node positive breast cancer (TAILOR RT) ClinicalTrials.gov. Updated November 10, 2020. Accessed December 22, 2020. https://clinicaltrials.gov/ct2/show/NCT03488693
- Jakubowski DM, Bailey H, Abran J, et al. Molecular characterization of breast cancer needle core biopsy specimens by the 21-gene Breast Recurrence Score test. J Surg Oncol. 2020;122(4):611-618. doi:10.1002/jso.26050
- Cortazar P, Zhang L, Untch M, et al. Pathological complete response and long-term clinical benefit in breast cancer: the CTNeoBC pooled analysis. Lancet. 2014;384(9938):164-172. doi:10.1016/S0140-6736(13)62422-8
- Gianni L, Zambetti M, Clark K, et al. Gene expression profiles in paraffin-embedded core biopsy tissue predict response to chemotherapy in women with locally advanced breast cancer. J Clin Oncol. 2005;23(29):7265-7277. doi:10.1200/JCO.2005.02.0818
- Pease AM, Riba LA, Gruner RA, Tung NM, James TA. Oncotype DX recurrence score as a predictor of response to neoadjuvant chemotherapy. Ann Surg Oncol. 2019;26(2):366-371. doi:10.1245/s10434-018-07107-8
- Yardley DA, Peacock NW, Shastry M, et al. A phase II trial of ixabepilone and cyclophosphamide as neoadjuvant therapy for patients with HER2-negative breast cancer: correlation of pathologic complete response with 21-gene recurrence score. Breast Cancer Res Treat. 2015;154(2):299-308. doi:10.1007/s10549-015-3613-y
- Spring LM, Gupta A, Reynolds KL, et al. Neoadjuvant endocrine therapy for estrogen receptor-positive breast cancer: a systematic review and meta-analysis. JAMA Oncol. 2016;2(11):1477-1486. doi:10.1001/jamaoncol.2016.1897
- Bear HD, Wan W, Robidoux A, et al. Using the 21-gene assay from core needle biopsies to choose neoadjuvant therapy for breast cancer: a multicenter trial. J Surg Oncol. 2017;115(8):917-923. doi:10.1002/jso.24610. Published correction appears in J Surg Oncol. 2018;118(4):722.
- Cardoso F, van’t Veer LJ, Bogaerts J, et al; MINDACT Investigators. 70-gene signature as an aid to treatment decisions in early-stage breast cancer. N Engl J Med. 2016;25(8):717-729. doi:10.1056/NEJMoa1602253
- Park SJ, Lee MH, Kong S-Y, et al. Use of adjuvant chemotherapy in hormone receptor-positive breast cancer with or without the 21-gene expression assay. Breast Cancer Res Treat. 2018;170(1):69-76. doi:10.1007/s10549-018-4740-z
Corresponding author:
Tari A. King, MD
Division of Breast Surgery
Dana-Farber/Brigham and Women’s Cancer Center
Address: Yawkey Center for Cancer Care, 450 Brookline Ave, Boston, MA 02215
Phone: 617-632-3891
Email: tking7@bwh.harvard.edu
Conflict of Interest Statement:
TAL, ACG-C and PDP have no disclosures.
EPW has received an honorarium from Genentech, USA Inc. EAM has the following disclosures: research support from GlaxoSmithKline; an honorarium from Physicians’ Education Resource; compensated participation on the scientific advisory board for Exact Sciences (formerly Genomic Health), Astra-Zeneca, Merck, Peregrine Pharmaceuticals, Roche/Genentech, Sellas Lifesciences, and TapImmune Inc; uncompensated service on steering committees for BMS, Lilly, and Roche/Genentech; institutional clinical trial funding from AstraZeneca, EMD Serono, Galena Biopharma, and Roche/Genentech at the MD Anderson Cancer Center and from Roche/Genentech via SU2C grant at the Dana-Farber Cancer Institute.
EAM also serves in nonremunerated positions on the Board of Directors for the American Society of Clinical Oncology and as a Scientific Advisor for the Susan G. Komen for the Cure Foundation.
TAK has the following disclosures: speakers’ honoraria and compensated participation on the scientific advisory board for Exact Sciences (formerly Genomic Health).
Funding Sources: EAM acknowledges Rob and Karen Hale Distinguished Chair in Surgical Oncology for support.
**Author Contributions: All authors contributed to the work’s conception, literature review, and drafting of the manuscript.

