Large Herceptin Trials Change the Standard of Care for HER2+ Breast Ca
ORLANDO-Interim findingsfrom two large randomized phaseIII studies-a North American jointanalysis and an international trial-have changed the standard of care for
ORLANDO-Interim findingsfrom two large randomized phaseIII studies-a North American jointanalysis and an international trial-have changed the standard of care forwomen with early invasive HER2-positive breast cancer. Trastuzumab(Herceptin) plus standard adjuvantchemotherapy yielded dramatic responses,cutting the risk of locoregionaland distant recurrence in halfand significantly improving overallsurvival, with acceptable cardiac tolerability.The unprecedented findingswere reported in a late-breaking scientificsymposium at ASCO.US and International TrialsThe joint analysis combined datafrom 3,351 patients in two NCI-sponsoredtrials; NSABP (National SurgicalAdjuvant Breast and BowelProject)-B31 and NCCTG (NorthCentral Cancer Treatment Group)-N9831; each study evaluated doxorubicin(Adriamycin) and cyclophosphamide(AC), followed by paclitaxel(Taxol), with or without trastuzumab,using different schedules of paclitaxel.HERA (HERceptin Adjuvant), a39-country, 5,090-patient study, assessedHerceptin vs observation followinga wide range of primary chemotherapyoptions, and radiotherapyas applicable; it is one of the largeststudies ever conducted in patients withbreast cancer.Trastuzumab has shown benefit butposes a small cardiac risk, and LVEF(left ventricular ejection fraction) wasmonitored in both the US and internationaltrials, with predeterminedtrastuzumab-stopping rules applied.
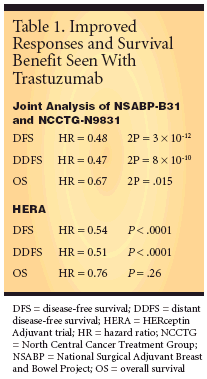
Results Publicized EarlyEfficacy results of the NSABP-B31/NCCTG-N9831 joint analysis and ofHERA were made public in April, andboth trials were stopped with about 2-year and 1-year median follow-up,respectively, because they had mettheir efficacy endpoints, with strikingoutcomes (Table 1).NSABP/NCCTG Joint AnalysisNSABP-B31 and NCCTG-N9831were led by NSABP and NCCTG, withCALGB (Cancer and Leukemia GroupB), ECOG (Eastern Cooperative OncologyGroup), and SWOG (SouthwestOncology Group). Because thetwo trial designs were so similar, theFDA agreed the data could be combinedfor a joint analysis.The study arm for the joint analysiswas AC followed by paclitaxel plustrastuzumab, and the control arm wasAC followed only by paclitaxel. TheNCCTG-N9831 had three study arms,one of which included Herceptin givensequentially with paclitaxel and sowas excluded from the joint analysis.NSABP study chair EdwardRomond, MD, associate professor ofmedicine at the University of KentuckyCollege of Medicine, Lexington,presented the joint-analysis findings.NCCTG chair Edith Perez, MD, presentedfurther analysis of NCCTGN9831,and in a poster presentationshe discussed interim cardiac safetydata from that trial (abstract 556). Dr.Perez is professor of medicine, MayoMedical School, director, Clinical Investigations,and director of the BreastCancer Program, in the division ofhematology/oncology at the MayoClinic, Jacksonville, Florida.Standard-Changing FindingsTrastuzumab was associated with a52% reduction in overall risk of localrecurrence at 3 years.Absolute reduction in risk of recurrenceby Kaplan-Meier analysis was12% with trastuzumab at 3 years and18% at 4 years. Time to first distantrecurrence was also assessed, as a sur-rogate for overall survival, and showedtrastuzumab plus chemotherapy reducedoverall probability of distantmetastases by 53% at 3 years. Estimatedabsolute reduction in risk with trastuzumabwas 9% at 3 years and 16% at4 years.At a median overall follow-up of 2years, trastuzumab conferred a 33%reduction in mortality. Estimated absolutereduction in risk of death withtrastuzumab was 2.5% at 3 years and4.8% at 4 years. "We have changed thestandard of care," Dr. Perez told ONI."Patients following resection shouldhave chemotherapy plus Herceptin tominimize the risk that cancer couldreturn."Acceptable Cardiac RiskAlthough chemotherapy of the typegiven in the NCCTG and NSABP trialscarries a congestive heart failure (CHF)risk less than 1%, in the joint analysisrisk of CHF in women receiving chemotherapyplus trastuzumab was increasedby 3% to 4%. In the separatecardiac safety analysis of NCCTGN9831,a 2.2% greater incidence ofcardiac events vs control was seen withsequential therapy and a 3.3% increasedincidence was seen with concurrenttherapy, but at this point inthe analysis the confidence intervalsoverlap, Dr. Perez said.Drs. Romond and Perez emphasizedthe importance of cardiac monitoringin all patients if trastuzumab isto be used in this regimen in the adjuvantsetting. However, in her analysisof NCCTG-N9831, Dr. Perez commentedthat although "adding trastuzumabto paclitaxel in the adjuvantsetting resulted in an increase in cardiactoxicity, this increase remains lessthan the threshold of 4% [above thatin the non-trastuzumab regimen], indicatingacceptable cardiac tolerability."She noted that a subset of patientswill be studied to evaluate the potentialrole of circulating biomarkers forpredicting or managing adverse cardiaceffects of trastuzumab.HERA StudyHERA evaluated duration of trastuzumabtreatment in women withearly-stage HER2-positive invasivebreast cancer, most of whom had receivedan anthracycline but not a taxane.About one-third were node-negative. Conducted by investigators fromRoche and the Breast InternationalGroup (BIG), it evaluated adjuvantHerceptin given every 3 weeks for 12or 24 months vs observation followingstandard adjuvant chemotherapygiven before or after surgery.HERA accrued 5,090 women at 478centers in 39 countries who had centrallyconfirmed HER2-positive invasivebreast cancer and had undergonesurgery with neoadjuvant chemotherapy,with or without radiotherapy.The findings were presented by leadinvestigator and chair of BIG MartineJ. Piccart-Gebhart, MD, PhD, associateprofessor of oncology at the Uni-versitLibre de Bruxelles and head ofthe department of medicine at the JulesBordet Institute, Brussels, Belgium.The interim analysis at 475 diseasefreesurvival events in 3,387 womencompared Herceptin vs observationbut not 12 months vs 24 months oftherapy; these data are not mature butwill become available later in the study.Dramatic ResultsHerceptin conferred a statisticallysignificant improvement in diseasefreesurvival, with a 46% reduction inlikelihood of recurrence (HR = 0.54, P< .0001), translating to an absoluteimprovement in 2-year disease-freesurvival of 8%. Relapse-free and distantdisease-free survival results "werequite remarkable," Dr. Piccart-Gebhartreported, "with a 50% reductionin risk, translating again into an 8% 2-year reduction in risk" (P < .0001).The secondary endpoint of overall survivalhas not yet been reached, possiblyowing to short follow-up, with 37deaths in the observation group and29 in the 12-month trastuzumabgroup.Results regarding optimal durationof therapy (ie, 12-month vs 24-monthtrastuzumab) will be available in 2008.Low Incidence of CHFIncidence of CHF was 0.5% in the12-month trastuzumab group vs 0 forpatients randomized to observation.The HERA study has an external independentdata monitoring committee(IDMC) that regularly reviews safetydata. No safety concerns were raisedby the IDMC, but patients will be followedfor side effects.