Lung Cancer in ‘Never-Smokers’: A Unique Entity
Lung cancer in “never-smokers” constitutes only a small proportion of patients with lung cancer. Nevertheless, the topic has recently attracted a good deal of attention. Initially this was due to the fact that never-smokers with lung cancer had better outcomes with epidermal growth factor receptor–tyrosine kinase (EGFR-TK) inhibitors, compared to tobacco smokers with lung cancer. More recently the identification of molecular changes unique to lung cancer in never-smokers has generated further interest in this disease. These findings have the potential to enhance our knowledge of lung cancer biology and lead to the development of new, more effective treatments for lung cancer. In this review, we summarize the existing body of knowledge on lung cancer in never-smokers.
Lung cancer in “never-smokers” constitutes only a small proportion of patients with lung cancer. Nevertheless, the topic has recently attracted a good deal of attention. Initially this was due to the fact that never-smokers with lung cancer had better outcomes with epidermal growth factor receptor–tyrosine kinase (EGFR-TK) inhibitors, compared to tobacco smokers with lung cancer. More recently the identification of molecular changes unique to lung cancer in never-smokers has generated further interest in this disease. These findings have the potential to enhance our knowledge of lung cancer biology and lead to the development of new, more effective treatments for lung cancer. In this review, we summarize the existing body of knowledge on lung cancer in never-smokers.
Lung cancer continues to be a leading cause of cancer-related mortality in the United States and globally. In 2009, it is estimated that more than 200,000 patients were diagnosed with lung cancer.[1] Non–small-cell lung cancer (NSCLC) comprises more than 85% of all lung cancers,[2] and tobacco smoking is the leading cause of NSCLC. Recently it was reported that patients with lung cancer who are lifelong “never-smokers” have better response rates with epidermal growth factor receptor–tyrosine kinase (EGFR-TK) inhibitors than smokers with lung cancer. This has led to an increased research focus on never-smokers with lung cancer, resulting in rapid growth of our knowledge on the outcomes and tumor biology of this disease. In the United States, the age-adjusted incidence rate for lung cancer in never-smokers aged 40 to 79 years ranges from 4.8 to 13.7 per 100,000 person-years for men and 14.4 to 20.8 per 100,000 person-years for women.[3] This review summarizes some of the recent and relevant findings on lung cancer in never-smokers.
Risk Factors
No single major risk factor for the development of lung cancer in never-smokers has been identified. Several potential risk factors have been studied including environmental exposures such as environmental tobacco smoke (ETS), radon, asbestos, cooking fumes, genetic susceptibility, hormonal factors, and oncogenic viruses.
Environmental Exposures
• Environmental Tobacco Smoke-Environmental tobacco smoke (ETS) is a combination of sidestream tobacco smoke (smoke from the end of a smoldering cigarette) and the mainstream tobacco smoke exhaled by the smoker. The US Surgeon General’s report published in 2006 stated that ETS increased the risk of lung cancer in never-smokers by 20% to 30%, based on a pooled analysis of more than 100 previously published studies. ETS as a risk factor for lung cancer in never-smokers possibly accounts for only a small proportion of patients.
• Radon-Radon is a uranium degeneration product that is known to cause lung cancer in uranium miners.[4] The risk for lung cancer from nonoccupational radon exposure has been evaluated by several large cohort and case-control studies in the United States. A pooled analysis of all the major North American case-control studies, with a total of 4,081 cases and 5,281 controls, revealed that the odds for developing lung cancer increased with residential radon concentration (excess odds ratio = 0.10 per 100 Bq/m3; 95% confidence interval [CI] = –0.01 to 0.26).[5] When the data analysis was restricted to never-smokers, the risk for lung cancer increased with radon concentration (excess odds ratio = 0.09 per 100 Bq/m3; 95% CI = –0.01 to 0.26).
• Asbestos-Nonoccupational asbestos exposure as a risk factor for lung cancer was studied in women from mining communities in Quebec. There was no increased mortality in this population (standardized proportionate mortality ratio = 1.1; 95% CI = 0.88–1.38).[6] In a more recent study from Australia, nonoccupational exposure to asbestos in women identified increased risk of death from lung cancer with a standardized mortality ratio of 2.15 (95% CI = 1.45–3.07).[7] Variations in geography as well as in type and amount of asbestos exposure could account for some of the conflicting data.
• Cooking Fumes-Exposure to cooking oil fumes and coal burning has been studied extensively in Chinese women with lung cancer. In a case-control study of 672 women with lung cancer and 735 healthy controls, exposure to rapeseed oil fumes was associated with increased risk for lung cancer (relative risk [RR] = 2.6; 95% CI = 1.3–5.1).[8] Coal fumes from cooking and indoor heating have been reported to be associated with increased risk of lung cancer in the Chinese population. In a case-control study of 846 lung cancer patients and 1,740 population controls from rural China, exposure to indoor coal fumes over a period of 30 years was associated with an increased risk for lung cancer (odds ratio = 1.29; 95% CI = 1.03–1.61).[9] Several other case-control studies have confirmed the increased risk for lung cancer in never-smokers exposed to cooking oil fumes and coal fumes (reviewed in reference 10).
Well known environmental risk factors such as ETS account for a small proportion of lung cancer cases in never-smokers. The risk of lung cancer in never-smokers from nonoccupational radon and asbestos exposure appears to be minimal. Exposure to cooking oil and coal fumes has been studied predominantly in the Chinese population and other Pacific Rim countries. It may have a wider role in other developing nations, although data are lacking. This factor is unlikely to be an issue in developed countries.
Genetic Susceptibility
The role of family history as a risk factor for lung cancer in never-smokers has been the focus of several studies. Analysis of 11 studies identified family history of lung cancer to be associated with an increased risk of developing lung cancer in never-smokers, with a pooled RR estimate of 1.51 (95% CI = 1.11–2.06).[11] Two genome-wide association studies of more than 3,000 cases and controls identified two single-nucleotide polymorphisms (SNPs) on chromosome 15q25 (rs1051730 and rs8034191) to be associated with an increased risk of developing lung cancer.[12,13] Both studies concluded that in never-smokers, these SNPs were not associated with an increased risk of lung cancer. However, these findings are limited by the small number of never-smokers compared to the entire study population (352 and 125 patients in the two studies, respectively).
Polycyclic amino hydrocarbons (PAH) in tobacco smoke are metabolized in a two-phase process, initially activated by the cytochrome P450 enzymes (CYPs) and then detoxified by glutathione-S-transferases (GSTs). In a pooled analysis of 14 case-control studies consisting of 302 never-smokers with lung cancer and 1,631 never-smoking controls without lung cancer, the risk of lung cancer was significantly increased if the CYP1A1 Ile(462)Val polymorphism was present (odds ratio = 2.99; 95% CI = 1.51–5.91).[14]
The association between lung cancer risk and alterations in DNA repair pathways has received wide attention. X-ray cross-complementing group 1 (XRCC1) is a DNA repair protein involved in base excision repair of DNA base damage and single-strand breaks. Evidence suggests that XRCC1 Arg399Gln polymorphism is associated with deficient DNA repair.[15] In a case-control study of 1,091 Caucasians with lung cancer and 1,240 healthy controls, the risk of lung cancer was increased in never-smokers with the XRCC1 Arg399Gln polymorphism (odds ratio = 2.4; 95% CI = 1.2–5.0).[16] In a larger study, the risk for lung cancer in never-smokers with XRCC1 Arg399Gln polymorphism was not significant (odds ratio = 0.83; 95% CI = 0.46–1.48).[17]
The increased risk for lung cancer in never-smokers with a family history of lung cancer indicates the existence of an inherited risk factor. Progress has been made in identifying genetic variants associated with increased risk for lung cancer. The identification of the SNPs rs1051730 and rs8034191 on 15q25 as a risk factor for lung cancer is an important advance in this field. At present it is not clear whether these SNPs are associated with lung cancer because they affect tobacco-smoking behavior or they directly increase the risk of lung cancer by another mechanism.[18] Polymorphisms of genes involved in xenobiotic metabolism and DNA repair have been extensively investigated. CYP1A1 Ile(462)Val polymorphism has now been shown in several studies to be associated with increased risk for lung cancer in never-smokers.[10] Polymorphisms of DNA repair genes require further investigation to identify their role in the development of lung cancer in never-smokers.
Oncogenic Virus
Human papillomavirus (HPV) is well known to be associated with cervical and tonsillar cancer. In a recent review of 53 studies from all over the world, the mean incidence of HPV in lung cancer tumor tissue was reported to be 24.5%.[19] Asians with lung cancer have a higher mean incidence of HPV in tumor tissue (36%) than patients with lung cancer from Europe (17%) and United States (15%). HPV 16/18 expression was detected by nested polymerase chain reaction (PCR) in 77 of 141 lung cancer tumor samples from Taiwan.[20] The detection rate was significantly higher in never-smokers with lung cancer than in tobacco smokers with lung cancer; for HPV 16 it was 48.7% vs 29% and for HPV 18 it was 57.3% vs 20.6%, respectively (P < .001). Subsequent studies from China fail to confirm the higher prevalence of HPV in never-smokers.[21,22] At the present time, an association between HPV and never-smokers with lung cancer remains unproven.
Hormonal Factors
Never-smokers with lung cancer are more likely to be women than men. Estrogen receptors ER-alpha and ER-beta are expressed in both normal and cancerous lung tissue.[23,24] Activation of these receptors by estrogen has been shown to promote proliferation of both normal and tumor cells.[24] Metabolic products of estrogen may cause DNA damage by forming DNA adducts, thereby promoting carcinogenesis.[25]
Epidemiologic studies have examined the role of estrogen as a risk factor for lung cancer. In a case-control study with 180 women with lung cancer and 303 controls from the United States, estrogen replacement therapy (ERT) was associated with increased risk for lung adenocarcinoma in smokers (odds ratio = 32.4; 95% CI = 15.9–665.3) but not in never-smokers (odds ratio = 1.0; 95% CI = 0.3–3.8).[26] However, other studies have reported no association between ERT and lung cancer (reviewed in reference 10). At present, there is no definitive evidence linking estrogen with increased risk for lung cancer. However, the role of sex hormones requires further investigation since never-smokers with lung cancer are more likely to be women than men.
Molecular Genetics of Never-Smokers With Lung Cancer
Attempts have been made to identify the underlying biologic differences between never-smokers with lung cancer and lung cancer in tobacco smokers. Various methods including gene-expression profiling, chromosomal copy number variations, individual gene mutations, and epigenetic profiling have been used in these studies (Table 1).
TABLE 1
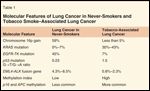
Molecular Features of Lung Cancer in Never-Smokers and Tobacco Smoke–Associated Lung Cancer
Whole-Genome Expression Profiling
Comparison of gene-expression profile of tumor tissue between smokers and never-smokers with lung cancer using microarray technology has been attempted in small samples. Two studies with small sample sizes reported no significant difference in the unsupervised hierarchical clustering between never-smokers and tobacco smokers with lung cancer.[27,28] They did identify several genes that were differentially expressed between these two groups; however, the exact significance of these genes is not understood at this time.
Unsupervised hierarchical clustering of whole-genome expression in a large sample (n = 90) of adenocarcinomas (included both smokers and never-smokers) identified two distinct subgroups.[29] One of these subgroups had pathologic features consistent with terminal respiratory unit (TRU) type adenocarcinoma. The TRU type adenocarcinoma cluster had a greater proportion of never-smokers than smokers (37 vs 8; P < .001).TRU type adenocarcinoma is characterized by lepidic growth, thyroid transcription factor 1 (TTF-1) and surfactant protein expression. It is also associated with a higher prevalence of EGFR-TK mutation compared to non–TRU type adenocarcinoma.
Chromosomal Aberrations
Comparative genomic hybridization (CGH) analysis of 32 never-smokers with adenocarcinoma reported gain at 16p (59%) as the most common chromosomal aberration in this cohort.[30] Further analysis in an unmatched group of smokers (n = 10) with adenocarcinoma identified gain in 16p in only 1 sample. This finding is yet to be confirmed independently.
p53
Mutations involving the tumor-suppressor gene p53 are common in many different types of cancer. They are reported to occur in 40% to 60% of all patients with NSCLC (reviewed in reference 31). p53 mutations are more common in tobacco smokers with lung cancer (26%–71%) than never-smokers with lung cancer (8%–47%).[31]
In addition to being more common in tobacco smokers with lung cancer than never-smokers with lung cancer, the type of mutation differs between the two groups. In tobacco smokers with lung cancer, the p53 mutation has a higher frequency of transversions than transitions.[32] A purine (G or A) is substituted by a pyrimidine (C or T) in tranversions. On the other hand, in transitions, a purine is replaced by another purine or a pyrimidine by another pyrimidine. In a cohort of 440 tobacco smokers with lung cancer and 156 never-smokers with lung cancer, G→T transversion mutations where reported in 30% of tobacco smokers with lung cancer vs 15% in never-smokers with lung cancer.[33] G→ A transition mutations were more frequent in never-smokers than smokers with lung cancer (44% vs 29%).
EGFR-TK and KRAS Mutations
It is now well established that EGFR-TK inhibitors produce dramatic and more durable benefit in patients with activating EGFR-TK mutation compared to those who do not.[34] A review of published studies that included 2,128 patients reported that the frequency ofEGFR-TK mutations in never-smokers with lung cancer was 45%, compared to only 7% in smokers with lung cancer.[35]
The KRAS oncogene mutation is more common in smokers with lung cancer and rare in never-smokers with lung cancer (reviewed in reference 31). In one study of 106 patients with adenocarcinoma, the frequency of KRAS mutation was higher in smokers than in never-smokers (43% vs 0%; P = .001).[36] It is uncommon for both KRAS mutation and EGFR-TK mutation to occur in the same patient. Both genes play an important role in cell proliferation, and the KRAS gene is downstream from the EGFR gene in the pathway. These findings led to the hypothesis that in a subset of never-smokers with lung cancer, the carcinogenesis pathway is driven by the EGFR-TK mutation (reviewed in reference 31).
EML4-ALK Fusion Gene
Mutations involving oncogenes and tumor-suppressor genes are a common feature in solid malignancies. In contrast, gene translocations resulting in the formation of fusion genes, although well recognized in hematologic malignancies, are uncommon in solid malignancies. The formation of echinoderm microtubule-associated protein-like 4 (2p21)–anaplastic lymphoma kinase (2p23), or the EML4-ALK fusion gene, due to chromosome 2 inversion was initially reported in Japanese patients with NSCLC. [37] The EML4-ALK fusion gene is reported to be associated with gain-of-function properties, although the exact role of activated ALK kinase in lung cancer is not well characterized. ALK was first discovered as a fusion protein with nucleophosmin in anaplastic large cell lymphoma with translocation (2;5).[38]
The EML4-ALK fusion gene is present in 3% to 7% of all patients with NSCLC (reviewed in reference 39). In a study from Hong Kong, reverse-transcription PCR on 240 resected primary NSCLC tumors identified EML4-ALK fusion in 13 samples.[40] Never-smokers with lung cancer were more likely to have the EML4-ALK fusion gene (10 of 115) than tobacco smokers with lung cancer (1 of 74, P = .013). Furthermore, patients with the EML4-ALK fusion gene had wild-type EGFR and KRAS genes. It appears that the EML4-ALK fusion gene and EGFR-TK mutation are mutually exclusive.[40]
More recently, 141 formalin-fixed paraffin-embedded NSCLC tumor samples from the United States were tested by fluorescence in-situ hybridization for the EML4-ALK fusion gene.[41] The fusion gene was detected in 19 (13%) of 141 tumor samples. The higher proportion of tumors with the EML4-ALK fusion gene could be due to the fact that only patients with at least two or more of the following criteria were included in the study: Asian ethnicity, female gender, never-smoker (< 100 cigarettes per life time) or light smoker (≤ 10 pack-years per lifetime), and adenocarcinoma histology. Compared to patients with wild-type ALK and EGFR, patients with the EML4-ALK fusion gene were younger (median age = 52 years vs 64 years; P = .005) and more likely to be men (58% vs 32%; P = .04) and never-smokers (74% vs 26%; P < .001). There was no difference in response to chemotherapy and overall survival between the wild-type and EML4-ALK fusion gene cohort.
Cell line experiments with NSCLC cells harboring the EML4-ALK fusion gene experienced cell death when treated with an ALK inhibitor.[42] Drugs targeting ALK are currently under development, and it would be interesting to see if these drugs might have a role in treating patients with NSCLC who carry the EML4-ALK fusion gene.
Methylation
Gene silencing of tumor-suppressor genes by methylation of CpG islands in the promoter sequence has been reported in lung cancer. Methylation of p16 and APC genes has been reported to be lower in never-smokers than in smokers with adenocarcinoma.[43] In a study involving 514 patients with NSCLC (with 112 never-smokers), the methylation index was lower in never-smokers than in tobacco smokers (P = .002). In another study, methylation of the MGMT gene was more frequent in never-smokers (n = 46) than in smokers (n = 157) with adenocarcinoma (66% vs 47%, P = .002).[44] However, this finding has not been confirmed by others.[43,45]
In patients with NSCLC, there are significant differences in the expression of specific molecular markers based on smoking status. These molecular changes have immediate implications as treatment selection is based on the biomarker profile. More work is needed to identify and characterize the molecular genetics of lung cancer in never-smokers.
Clinical Features
Presentation and Outcomes
TABLE 2

Clinical Features of Lung Cancer in Never-Smokers and Tobacco Smoke–Associated Lung Cancer
Several retrospective studies comparing the clinical features and outcomes of lung cancer in never-smokers to tobacco smokers with lung cancer have been published.[3,46,47] These studies have uniformly reported a higher proportion of women and adenocarcinoma histology in patients with lung cancer who are lifelong never-smokers compared to those with a history of tobacco smoking and lung cancer (Table 2).[10] Studies from Asia report a higher proportion of stage IV disease in never-smokers with lung cancer than tobacco smokers with lung cancer,[48,49] but such differences have not been consistently reported in other studies.[46,50]
Studies have reported better outcomes for lung cancer in never-smokers compared to smokers with lung cancer.[46,48,50] In a study from Singapore that included 975 patients with NSCLC, the 5-year survival rate for never-smokers (n = 286) was 23% compared to 16% in smokers with lung cancer (P = .02). However, other studies have reported no significant difference in the outcomes for patients with NSCLC based on their smoking status.[47,51] Our experience based on a matched pair analysis of never-smokers and tobacco smokers with lung cancer showed no significant difference in outcomes between the two groups-the 5-year survival rate was 27.2% vs 31.3%, respectively (P = .73).[47]
Response to Chemotherapy
Few studies have reported on the effect of cytotoxic chemotherapy on outcomes for patients with NSCLC based on smoking status. A retrospective study of 1,370 patients with stage III/IV NSCLC undergoing treatment with chemotherapy and radiation or chemotherapy alone reported better 1-year survival for never-smokers undergoing chemotherapy than smokers with NSCLC (62.6% vs 42.7%; P < .0001).[52] However, results from the prospective phase III Tarceva Responses in Conjunction with Carboplatin and Paclitaxel (TRIBUTE) trial did not identify better outcomes in never-smokers vs tobacco smokers with lung cancer; median overall survival was 10.1 vs 9.1 months, respectively.[53]
Never-smokers with lung cancer are characterized by a higher proportion of women and adenocarcinoma than their smoking counterparts. It appears that they are more likely to present with distant-stage disease. The data on survival outcomes for never-smokers and tobacco smokers with lung cancer are conflicting, and treatment with cytotoxic chemotherapy alone may not lead to better outcomes in never-smokers with lung cancer (reviewed in reference 10). Furthermore, the better outcomes reported in never-smokers with lung cancer in some studies may be attributed to the higher incidence of comorbidities in tobacco smokers with lung cancer.
Response to EGFR-TK Inhibitors
Patients with NSCLC who are positive for activating EGFR-TK mutations have better outcomes to treatment with EGFR-TK inhibitors than patients with wild-type EGFR.[34] It has also been reported that never-smokers are more likely to harbor activating EGFR-TK mutations than smokers with NSCLC (reviewed in reference 10).
Reference Guide
Therapeutic Agents
Mentioned in This Article
Carboplatin
Erlotinib (Tarceva)
Gefitinib (Iressa)
Gemcitabine (Gemzar)
Paclitaxel
Brand names are listed in parentheses only if a drug is not available generically and is marketed as no more than two trademarked or registered products. More familiar alternative generic designations may also be included parenthetically.
Several large phase III trials have examined the role of EGFR-TK inhibitors in unselected patients with advanced NSCLC. The BR.21 trial reported a survival advantage for erlotinib (Tarceva) vs placebo in patients with relapsed or refractory NSCLC (hazard ratio [HR] = 0.7; 95% CI = 0.58–0.85).[54] In the subgroup analysis, never-smokers had better outcomes than smokers (HR = 0.8; 95% CI = 0.6–1). The Iressa Survival Evaluation in Lung Cancer (ISEL) trial did not meet its primary endpoint of improved survival in unselected patients with refractory NSCLC receiving gefitinib (Iressa) vs placebo.[55] Nevertheless, these investigators did report that never-smokers had better overall survival (median = 8.9 months) than tobacco smokers with lung cancer (median = 6.1 months; HR = 0.67; 95% CI = 0.49–0.92).
Combining erlotinib or gefitinib with cytotoxic chemotherapy in the front-line setting for patients with NSCLC has not proven to be successful.[53,56,57] Subgroup analysis from the phase III TRIBUTE trial reported better outcomes in never-smokers with NSCLC receiving erlotinib with carboplatin and paclitaxel than placebo with carboplatin and paclitaxel (HR = 0.49; 95% CI = 0.28–0.85).[53] In the Tarceva Lung Cancer Investigation Trial (TALENT), smoking history was available only in a small number of patients, and median overall survival was not reached (at the time of publication) for never-smokers (n = 8) in the erlotinib arm.[56] The progression-free survival for never-smokers receiving placebo with cisplatin and gemcitabine (Gemzar) was 5.5 months vs 7.9 months for patients receiving cisplatin and gemcitabine with erlotinib (HR = 0.195; P = .02).
The phase III Iressa Pan-Asia Study (IPASS) compared single-agent gefitinib to carboplatin and paclitaxel in chemotherapy-naive Asian patients with NSCLC.[58] The study included only patients with adenocarcinoma and never-smokers or former light smokers. The study enrolled 1,217 patients, and the primary endpoint was noninferiority of progression-free survival between the two arms. The patients in the gefitinib arm had better progression-free survival than patients receiving carboplatin and paclitaxel (HR = 0.74; 95% CI = 0.65–0.85). A planned subanalysis on the efficacy of EGFR biomarkers found that the survival benefit with gefitinib was limited to patients with the EGFR-TK mutation (HR = 0.48; 95% CI = 0.36–0.64). In the EGFR-TK mutation–negative cohort, patients receiving gefitinib had worse outcomes compared to patients on carboplatin and paclitaxel (HR = 2.85; 95% CI = 2.05–3.98).
The results of a planned subgroup analysis in the IPASS clinical trial show that the benefit for single-agent gefitinib in chemotherapy-naive patients is limited to EGFR-TK mutation-positive patients. In the future, patients with advanced NSCLC who are likely to harbor the EGFR-TK mutation (eg, never-smokers) may need to be screened for the mutation prior to starting treatment. Other options (mainly combination cytotoxic chemotherapy) will have to be considered for mutation-negative patients.
Results from the phase II Cancer and Leukemia Group B (CALGB) 30406 trial will hopefully provide further guidance on the role of erlotinib in the front-line setting for patients with advanced NSCLC. In this phase II study, treatment-naive patients with NSCLC who are never-smokers or former light smokers are randomized to receive either single-agent erlotinib or carboplatin and paclitaxel with erlotinib.
ALK Inhibitor
PF-02341066 is an orally administered small-molecule tyrosine kinase inhibitor with activity against c-Met and ALK receptors. In a phase I dose escalation study of PF-02341066, 37 patients with advanced cancer including 10 patients with NSCLC whose tumors were positive for the EML4-ALK rearrangement received the study drug.[59] Among patients with NSCLC, 1 confirmed partial response, 2 unconfirmed partial responses, and 4 patients with stable disease were reported. The maximum tolerated dose was 250 mg given twice daily, and major adverse effects were fatigue, nausea, vomiting, and diarrhea. These results show that PF-02341066 has activity in patients with advanced NSCLC who harbor the EML4-ALK rearrangement.
Conclusions
Epidemiologic evidence indicates that lung cancer in never-smokers is a distinct entity compared to the more common tobacco-associated lung cancer. The population of never-smokers with lung cancer is characterized by a higher proportion of women, adenocarcinoma histology, and Asian ethnicity. In addition, there are significant biologic differences reported between never-smokers and tobacco smokers with lung cancer. The identification of specific molecular markers that are more frequently identified in never-smokers (EGFR-TK mutations and EML4-ALK fusion gene) has immediate therapeutic implications. The identification of such molecular changes is critical for the development of new and effective diagnostic and treatment modalities for lung cancer patients.
Financial Disclosure:Dr. Govindan is a consultant for Bristol, Genentech, AstraZeneca, Boehringer-Ingelheim, and Eli Lilly.
References:
References
1. Jemal A, Siegel R, Ward E, et al: Cancer statistics, 2009. CA Cancer J Clin 59:225-249, 2009.
2. Govindan R, Page N, Morgensztern D, et al: Changing epidemiology of small-cell lung cancer in the united states over the last 30 years: Analysis of the surveillance, epidemiologic, and end results database. J Clin Oncol 24:4539-4544, 2006.
3. Wakelee HA, Chang ET, Gomez SL, et al: Lung cancer incidence in never smokers. J Clin Oncol 25:472-478, 2007.
4. Samet JM: Radon and lung cancer. J Natl Cancer Inst 81:745-757, 1989.
5. Krewski D, Lubin JH, Zielinski JM, et al: A combined analysis of North American case-control studies of residential radon and lung cancer. J Toxicol Environ Health A 69:533-597, 2006.
6. Camus M, Siemiatycki J, Meek B: Nonoccupational exposure to chrysotile asbestos and the risk of lung cancer. N Engl J Med 338:1565-1571, 1998.
7. Reid A, Heyworth J, de Klerk N, et al: The mortality of women exposed environmentally and domestically to blue asbestos at Wittenoom, Western Australia. Occup Environ Med 65:743-749, 2008.
8. Gao YT, Blot WJ, Zheng W, et al: Lung cancer among chinese women. Int J Cancer 40:604-609, 1987.
9. Kleinerman RA, Wang Z, Wang L, et al: Lung cancer and indoor exposure to coal and biomass in rural china. J Occup Environ Med 44:338-344, 2002.
10. Subramanian J, Govindan R: Lung cancer in never smokers: A review. J Clin Oncol 25:561-570, 2007.
11. Matakidou A, Eisen T, Houlston RS: Systematic review of the relationship between family history and lung cancer risk. Br J Cancer 93:825-833, 2005.
12. Hung RJ, McKay JD, Gaborieau V, et al: A susceptibility locus for lung cancer maps to nicotinic acetylcholine receptor subunit genes on 15q25. Nature 452:633-637, 2008.
13. Amos CI, Wu X, Broderick P, et al: Genome-wide association scan of tag SNPs identifies a susceptibility locus for lung cancer at 15q25.1. Nat Genet 40:616-622, 2008.
14. Hung RJ, Boffetta P, Brockmoller J, et al: Cyp1a1 and gstm1 genetic polymorphisms and lung cancer risk in caucasian non-smokers: A pooled analysis. Carcinogenesis 24:875-882, 2003.
15. Duell EJ, Wiencke JK, Cheng TJ, et al: Polymorphisms in the DNA repair genes xrcc1 and ercc2 and biomarkers of DNA damage in human blood mononuclear cells. Carcinogenesis 21:965-971, 2000.
16. Zhou W, Liu G, Miller DP, et al: Polymorphisms in the DNA repair genes xrcc1 and ercc2, smoking, and lung cancer risk. Cancer Epidemiol Biomarkers Prev 12:359-365, 2003.
17. Hung RJ, Brennan P, Canzian F, et al: Large-scale investigation of base excision repair genetic polymorphisms and lung cancer risk in a multicenter study. J Natl Cancer Inst 97:567-576, 2005.
18. Thorgeirsson TE, Geller F, Sulem P, et al: A variant associated with nicotine dependence, lung cancer and peripheral arterial disease. Nature 452:638-642, 2008.
19. Klein F, Amin Kotb WF, Petersen I: Incidence of human papilloma virus in lung cancer. Lung Cancer 65:13-18, 2009.
20. Cheng YW, Chiou HL, Sheu GT, et al: The association of human papillomavirus 16/18 infection with lung cancer among nonsmoking taiwanese women. Cancer Res 61:2799-2803, 2001.
21. Fei Y, Yang J, Hsieh WC, et al: Different human papillomavirus 16/18 infection in chinese non-small cell lung cancer patients living in wuhan, china. Jpn J Clin Oncol 36:274-279, 2006.
22. Lim WT, Chuah KL, Leong SS, et al: Assessment of human papillomavirus and epstein-barr virus in lung adenocarcinoma. Oncol Rep 21:971-975, 2009.
23. Canver CC, Memoli VA, Vanderveer PL, et al: Sex hormone receptors in non-small-cell lung cancer in human beings. J Thorac Cardiovasc Surg 108:153-157, 1994.
24. Stabile LP, Davis AL, Gubish CT, et al: Human non-small cell lung tumors and cells derived from normal lung express both estrogen receptor alpha and beta and show biological responses to estrogen. Cancer Res 62:2141-2150, 2002.
25. Yager JD, Liehr JG: Molecular mechanisms of estrogen carcinogenesis. Annu Rev Pharmacol Toxicol 36:203-232, 1996.
26. Taioli E, Wynder EL: Re: Endocrine factors and adenocarcinoma of the lung in women. J Natl Cancer Inst 86:869-870, 1994.
27. Miura K, Bowman ED, Simon R, et al: Laser capture microdissection and microarray expression analysis of lung adenocarcinoma reveals tobacco smoking- and prognosis-related molecular profiles. Cancer Res 62:3244-3250, 2002.
28. Powell CA, Spira A, Derti A, et al: Gene expression in lung adenocarcinomas of smokers and nonsmokers. Am J Respir Cell Mol Biol 29:157-162, 2003.
29. Takeuchi T, Tomida S, Yatabe Y, et al: Expression profile-defined classification of lung adenocarcinoma shows close relationship with underlying major genetic changes and clinicopathologic behaviors. J Clin Oncol 24:1679-1688, 2006.
30. Wong MP, Fung LF, Wang E, et al: Chromosomal aberrations of primary lung adenocarcinomas in nonsmokers. Cancer 97:1263-1270, 2003.
31. Subramanian J, Govindan R: Molecular genetics of lung cancer in people who have never smoked. Lancet Oncol 9:676-682, 2008.
32. Hainaut P, Pfeifer GP: Patterns of p53 g-->t transversions in lung cancers reflect the primary mutagenic signature of DNA-damage by tobacco smoke. Carcinogenesis 22:367-374, 2001.
33. Toyooka S, Tsuda T, Gazdar AF: The tp53 gene, tobacco exposure, and lung cancer. Hum Mutat 21:229-239, 2003.
34. Lynch TJ, Bell DW, Sordella R, et al: Activating mutations in the epidermal growth factor receptor underlying responsiveness of non-small-cell lung cancer to gefitinib. N Engl J Med 350:2129-2139, 2004.
35. Shigematsu H, Gazdar AF: Somatic mutations of epidermal growth factor receptor signaling pathway in lung cancers. Int J Cancer 118:257-262, 2006.
36. Shigematsu H, Lin L, Takahashi T, et al: Clinical and biological features associated with epidermal growth factor receptor gene mutations in lung cancers. J Natl Cancer Inst 97:339-346, 2005.
37. Soda M, Choi YL, Enomoto M, et al: Identification of the transforming EML4-ALK fusion gene in non-small-cell lung cancer. Nature 448:561-566, 2007.
38. Morris SW, Kirstein MN, Valentine MB, et al: Fusion of a kinase gene, alk, to a nucleolar protein gene, npm, in non-Hodgkin’s lymphoma. Science 263:1281-1284, 1994.
39. Horn L, Pao W: EML4-ALK: Honing in on a new target in non-small-cell lung cancer. J Clin Oncol 27:4232-4235, 2009.
40. Wong DW, Leung EL, So KK, et al: The EML4-ALK fusion gene is involved in various histologic types of lung cancers from nonsmokers with wild-type EGFR and KRAS. Cancer 115:1723-1733, 2009.
41. Shaw AT, Yeap BY, Mino-Kenudson M, et al: Clinical features and outcome of patients with non-small-cell lung cancer who harbor EML4-ALK. J Clin Oncol 27:4247-4253, 2009.
42. Koivunen JP, Mermel C, Zejnullahu K, et al: EML4-ALK fusion gene and efficacy of an alk kinase inhibitor in lung cancer. Clin Cancer Res 14:4275-4283, 2008.
43. Toyooka S, Maruyama R, Toyooka KO, et al: Smoke exposure, histologic type and geography-related differences in the methylation profiles of non-small cell lung cancer. Int J Cancer 103:153-160, 2003.
44. Pulling LC, Divine KK, Klinge DM, et al: Promoter hypermethylation of the o6-methylguanine-DNA methyltransferase gene: More common in lung adenocarcinomas from never-smokers than smokers and associated with tumor progression. Cancer Res 63:4842-4848, 2003.
45. Liu Y, Lan Q, Siegfried JM, et al: Aberrant promoter methylation of p16 and MGMT genes in lung tumors from smoking and never-smoking lung cancer patients. Neoplasia 8:46-51, 2006.
46. Nordquist LT, Simon GR, Cantor A, et al: Improved survival in never-smokers vs current smokers with primary adenocarcinoma of the lung. Chest 126:347-351, 2004.
47. Subramanian J, Velcheti V, Gao F, et al: Presentation and stage-specific outcomes of lifelong never-smokers with non-small cell lung cancer (NSCLC). J Thorac Oncol 2:827-830, 2007.
48. Toh CK, Gao F, Lim WT, et al: Never-smokers with lung cancer: Epidemiologic evidence of a distinct disease entity. J Clin Oncol 24:2245-2251, 2006.
49. Toh CK, Wong EH, Lim WT, et al: The impact of smoking status on the behavior and survival outcome of patients with advanced non-small cell lung cancer: A retrospective analysis. Chest 126:1750-1756, 2004.
50. Dibble R, Langeburg W, Bair S, et al: Natual history of non-small cell lung cancer in non-smokers (abstract 7252). J Clin Oncol 23(16S):683s, 2005.
51. Baser S, Shannon VR, Eapen GA, et al: Smoking cessation after diagnosis of lung cancer is associated with a beneficial effect on performance status. Chest 130:1784-1790, 2006.
52. Tsao AS, Liu D, Lee JJ, et al: Smoking affects treatment outcome in patients with advanced nonsmall cell lung cancer. Cancer 106:2428-2436, 2006.
53. Herbst RS, Prager D, Hermann R, et al: Tribute: A phase III trial of erlotinib hydrochloride (OSI-774) combined with carboplatin and paclitaxel chemotherapy in advanced non-small-cell lung cancer. J Clin Oncol 23:5892-5899, 2005.
54. Shepherd FA, Rodrigues Pereira J, Ciuleanu T, et al: Erlotinib in previously treated non-small-cell lung cancer. N Engl J Med 353:123-132, 2005.
55. Thatcher N, Chang A, Parikh P, et al: Gefitinib plus best supportive care in previously treated patients with refractory advanced non-small-cell lung cancer: Results from a randomised, placebo-controlled, multicentre study (Iressa Survival Evaluation in Lung Cancer). Lancet 366:1527-1537, 2005.
56. Gatzemeier U, Pluzanska A, Szczesna A, et al: Phase III study of erlotinib in combination with cisplatin and gemcitabine in advanced non-small-cell lung cancer: The Tarceva Lung Cancer Investigation trial. J Clin Oncol 25:1545-1552, 2007.
57. Giaccone G, Herbst RS, Manegold C, et al: Gefitinib in combination with gemcitabine and cisplatin in advanced non-small-cell lung cancer: A phase III trial--INTACT 1. J Clin Oncol 22:777-784, 2004.
58. Mok TS, Wu YL, Thongprasert S, et al: Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N Engl J Med 361:947-957, 2009.
59. Kwak EL, Camidge DR, Clark J, et al: Clinical activity observed in a phase I dose escalation trial of an oral c-met and ALK inhibitor, PF-02341066 (abstract 3509). J Clin Oncol 27(15S):148s, 2009.