Management of the Clinically Node-Negative Axilla: What Have We Learned From the Clinical Trials?
Here we review the evolution of sentinel lymph node biopsy for the management of clinically node-negative breast cancer, and we address the current controversies and management issues.
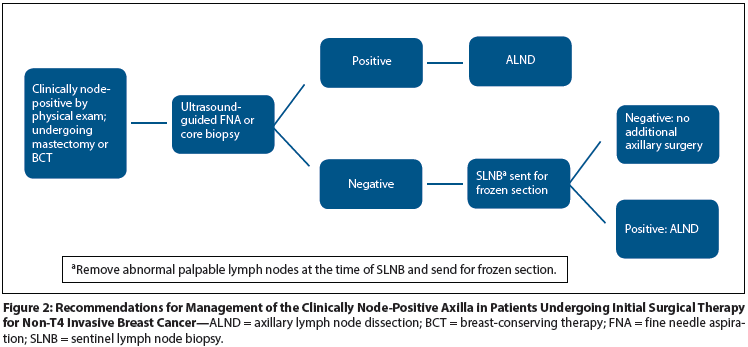
Table: Randomized Controlled Trials of Macrometastatic Axillary Disease Management
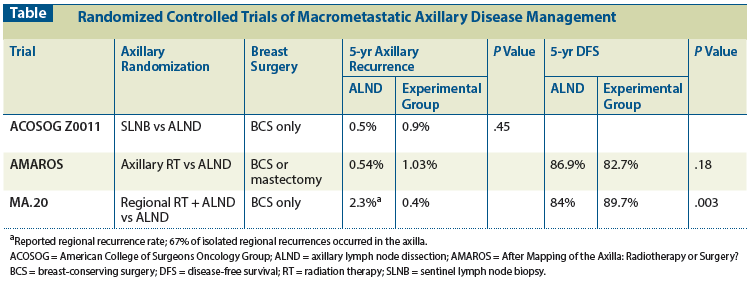
Figure 1: Recommendations for Management of the Clinically Node-Negative Axilla in Patients Undergoing Initial Surgical Therapy for Non-T4 Invasive Breast Cancer
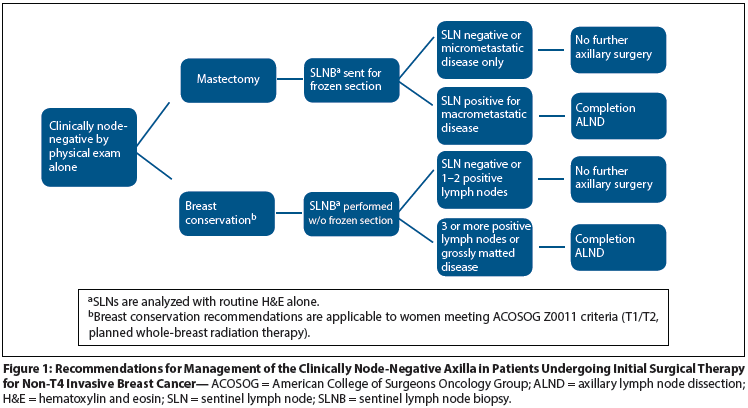
Figure 2: Recommendations for Management of the Clinically Node-Positive Axilla in Patients Undergoing Initial Surgical Therapy for Non-T4 Invasive Breast Cancer
Sentinel lymph node biopsy (SLNB) has revolutionized the surgical management of the axilla for patients with early breast cancer. SLNB initially became standard regional therapy for women who were both clinically and pathologically node-negative. Subsequently, SLNB has been established as appropriate management in patients with very low axillary tumor burden, defined as isolated tumor cells or micrometastatic disease (< 2 mm); it provides accurate staging information with no detriment to regional control. More recently, the treatment of the axilla has evolved for women with macrometastatic axillary disease. Three randomized controlled trials have compared different regional treatment strategies for patients with > 2 mm of axillary tumor burden. Here we review the evolution of SLNB for the management of clinically node-negative breast cancer, and we address the current controversies and management issues.
Introduction
Although axillary lymph node dissection (ALND) remained the standard of care for all breast cancer patients until the early 2000s, evidence that not all microscopic axillary disease requires surgical removal has been present for decades. The National Surgical Adjuvant Breast and Bowel Project (NSABP) B-04 trial established survival equivalence between clinically node-negative breast cancer treated with either total mastectomy alone with delayed axillary dissection for nodal recurrence, or total mastectomy with immediate axillary clearance. In these patients, who received no systemic or radiation therapy (RT), the subsequent axillary failure rate in the total-mastectomy-alone group (19%) was only about half that predicted from the proportion of patients with axillary metastases who underwent an ALND (40%).[1] Compared with the patients enrolled in this early trial, the breast cancer patients seen today not only have smaller cancers and lower nodal disease burden, but the majority of patients, even those with T1 tumors and pathologically negative nodes, are treated with adjuvant systemic therapy, which is now recognized to improve local control.[2] Decisions regarding the type of systemic therapy are rarely made based on the extent of axillary nodal disease, raising the question of whether ALND is necessary for local control or contributes to survival in patients with sentinel node metastases treated in the modern multidisciplinary era.
Sentinel Lymph Node Biopsy for Pathologically Negative Lymph Nodes
It is well documented that in women with clinically node-negative early breast cancer, a negative sentinel lymph node biopsy (SLNB) provides accurate staging information without increasing the risk of regional recurrence or decreasing survival. Veronesi et al, between 1998 and 1999, randomized women with clinical T1N0 breast cancer and a negative sentinel lymph node (SLN) to SLNB alone or SLNB followed by ALND. A total of 341 women with node-negative disease were included in the analysis. Of the women randomized to ALND, 5% (8/174) were found to have a false-negative SLNB. Among all eligible patients, there was 1 axillary recurrence at 7 years follow-up, in a patient treated with SLNB alone. The annual rate of events associated with breast cancer, including local, regional, and distant recurrence, did not differ between the groups (11.5 per 1,000 events in the ALND arm, and 9.8 per 1,000 events in the SLNB arm). Five-year disease-free survival (DFS) and overall survival (OS) were also similar (DFS: 88.9% in the ALND group vs 92.2% in the SLNB group, log-rank P = .2; OS: 96.4% vs 98.4%, respectively).[3] NSABP B-32, between 1999 and 2004, similarly randomized patients with clinically node-negative breast cancer and a negative SLN to SLNB alone or SLNB followed by ALND. Of these patients, 85% received systemic therapy and 82% received RT. There were no differences in local recurrence (P = .55), regional recurrence (P = .22), DFS (8-year estimates: 82% in the ALND group vs 82% in the SLNB group), or OS (8-year estimates: 92% in the ALND group vs 90% in the SLNB group). Importantly, both treatment arms experienced a < 1% rate of regional recurrence as first event in spite of a false-negative rate of 10% in patients who underwent ALND after SLNB.[4,5] A meta-analysis of 48 studies that included nearly 15,000 patients with SLN-negative breast cancer who did not have a subsequent ALND, with a median follow-up of 34 months, found an overall axillary recurrence rate of just 0.3%.[6] This axillary recurrence rate is substantially lower than the accepted false-negative rate of approximately 7%[7] for the procedure, demonstrating again that not all microscopic axillary disease becomes clinically apparent. Additionally, in two randomized studies that compared the identification of axillary nodal metastases with SLNB vs ALND alone,[8,9] no differences were observed, illustrating the point that while failure to identify the correct sentinel node or all of the sentinel nodes is associated with a false-negative rate of 5% to 10%, a similar false-negative rate occurs after ALND, which is due to the inability of the pathologist to perform a detailed examination of all the nodes in an ALND specimen.
The Axillary Lymphatic Mapping Against Nodal Axillary Clearance (ALMANAC) trial studied the morbidity and quality of life associated with SLNB compared with ALND. Women who underwent SLNB alone experienced significantly less lymphedema (relative risk [RR], 0.37) and less sensory loss (RR, 0.37) than women in the ALND group at 12 months. SLNB alone was associated with shorter time to resumption of normal daily activities (P < .001), as well as improved patient-recorded quality of life and arm functioning scores (P ≤ .003).[8] SLNB data showing similar survival outcomes and extremely low regional recurrence rates in conjunction with decreased postoperative morbidity compared with ALND established this less-invasive surgical procedure as standard staging and regional surgery for patients with a negative SLN.
Micrometastatic Disease
A consequence of the SLNB procedure is the ability to perform a more thorough evaluation of a smaller number of lymph nodes, resulting in an increased detection of micrometastases and isolated tumor cells (ITCs). Micrometastases are defined as a tumor deposit greater than 0.2 mm and/or more than 200 cells, but less than 2.0 mm, while ITCs are clusters of cells not greater than 0.2 mm or fewer than 200 cells.[10] A prospective comparison of axillary metastasis detection between routine ALND specimen evaluation and SLNs evaluated by serial sectioning followed by both hematoxylin and eosin (H&E) staining and cytokeratin immunohistochemistry (IHC) revealed significantly higher axillary metastatic detection rates in the SLNB group (42% in the SLNB group vs 29% in the ALND group; P < .03). Significantly fewer patients in the ALND-alone group had micrometastatic disease, defined as ≤ 2 mm of tumor burden, compared with those who also underwent SLNB with a combination of detection techniques (3% vs 16%, respectively; P < .0005).[11] The significance and surgical management of this low-volume SLN disease burden has since been studied.
Between 2001 and 2010, the International Breast Cancer Study Group (IBCSG) 23-01 trial recruited women from 27 institutions with a primary breast tumor ≤ 5 cm and only micrometastatic disease in an SLN; the women were randomized to SLNB alone or standard completion ALND. The trial closed early because of low accrual and lower-than-projected event rates (target accrual, 1,960; actual accrual, 934). SLN evaluation consisted of serial sectioning and either H&E staining or IHC. Patient and tumor characteristics included a median age of 54 years, 69% with T1 tumors, 90% with estrogen receptor (ER)-positive tumors, 91% treated with lumpectomy, and 96% receiving systemic therapy. Additional positive lymph nodes were identified in 13% of patients in the ALND arm. At a median follow-up of 5 years, the axillary recurrence rate was < 1% in both arms. Survival outcomes were similar in the ALND-alone and SLNB-alone groups (DFS: 84% vs 88%, respectively, log-rank P = .16; OS: 98% vs 98%, respectively, log-rank P = .73).[12]
Similarly, between 2001 and 2008, a multicenter randomized controlled trial in Spain compared ALND vs SLNB alone in 233 clinically node-negative patients with tumor size < 3.5 cm and an SLN with micrometastatic disease. SLNs were evaluated with serial sectioning and both routine H&E staining and IHC. Ninety-two percent of the women were treated with lumpectomy and 93% received adjuvant systemic therapy. At a median follow-up of 5.2 years, there were only four cases of any disease recurrence; these included two axillary nodal recurrences in the SLNB group and one axillary soft-tissue recurrence in the ALND arm. The overall DFS was 98%; there was no difference between the surgical arms (log-rank P = .33).[13] â©The above-mentioned trials, which resulted in similar regional recurrence and survival for patients treated with SLNB and those treated with ALND for micrometastatic disease, used both H&E staining and IHC to pathologically assess SLNs. The American College of Surgeons Oncology Group (ACOSOG) Z0010 trial was a prospective observational study that aimed to evaluate the prevalence and significance of IHC-detected occult micrometastases in SLNs and bone marrow in women with clinical T1/T2,N0 breast cancer. A total of 5,210 patients underwent breast-conserving surgery and SLNB, evaluated only by H&E staining. Of women with an identified SLN, 24% had H&E-detected positive axillary disease. From the remaining patients, 3,326 H&E-negative SLNs were sent to a central laboratory for IHC evaluation, and treating physicians were blinded to these results. Of the H&E-negative SLNs, 10.5% were found to contain IHC-detected occult metastases. At a median follow-up of 6.3 years, among women with H&E-negative SLNs, the presence of IHC evidence of occult metastasis had no significant association with recurrence or death: the 5-year DFS was 92% for the IHC-negative SLNs and 90% for the IHC-positive SLNs (P = .82).[14] The NSABP B32 trial also examined the prognostic significance of IHC-only SLN metastases. When results were initially reported at 5 years of follow-up, small but statistically significant decreases in DFS and OS were seen in patients with micrometastatic disease (DFS with occult metastases detected, 86%, vs with occult metastases not detected, 89%, P = .02; OS, 95% vs 96%, respectively, P = .03).[15] However, with 10 years of follow-up, although a 4% difference in DFS (P = .02) persisted, no statistically significant difference in OS was observed between the groups.[16]
These data highlight the fact that micrometastatic disease is safely managed with SLNB alone, and that the additional identification of IHC micrometastatic disease does not impact recurrence or OS; IHC is therefore not warranted. Updated National Comprehensive Cancer Network (NCCN) guidelines support this statement and note that SLN involvement is defined by multilevel nodal sectioning with H&E staining alone, with routine IHC not recommended.[10] A review of the National Cancer Database showed that over time, women with micrometastatic SLN metastases were increasingly managed with SLNB alone, with the proportion of such cases managed in this way increasing from 25% to 45% between 1998 and 2005 (P < .001).[17] Interestingly, the presence of ITCs or micrometastatic disease in the SLN does not guarantee the absence of macrometastatic disease in non-SLNs. Two meta-analyses reported additional non-SLN disease in 12% to 20% of women with ITCs or micrometastases in the SLN, and frequently, the additional disease consisted of macrometastases.[18,19] Given that macrometastatic disease is knowingly and safely left behind in the axilla of patients with micrometastatic SLN disease, the arbitrary cut-off of 2 mm of tumor burden (below which it is considered safe to omit an ALND) was questioned.
Macrometastatic Axillary Disease
Three trials have recently evaluated different treatment algorithms for patients with macrometastatic axillary disease. These trials are summarized in the Table; they differ in the targeted patient populations as well as in their experimental treatment arms.
ACOSOG Z0011
The ACOSOG Z0011 trial[20] randomized women with clinical T1/T2,N0 tumors with 1 or 2 positive SLNs detected by H&E only either to completion ALND or to observation. All women were treated with lumpectomy and whole-breast RT without axillary nodal irradiation. The trial was originally designed to include 1,900 patients but closed after accrual of 891 due to both slow accrual and a lower-than-projected event rate. Overall, 69% of tumors were T1, 83% of tumors were ER-positive, and 97% of women were treated with systemic therapy. Micrometastatic tumor burden accounted for 38% of positive nodes in the ALND arm and for 45% in the SLND arm. Twenty-seven percent of women in the ALND arm had additional positive nodes on evaluation of the axillary contents, so it is assumed that the SLNB group had a similar percentage of patients with axillary disease left behind. Regional recurrences occurred in < 1% in each group at a median follow-up of 6.3 years (0.9% in the SLNB group vs 0.5% in the ALND group; P = .45). Local recurrence at 5 years did not differ between the groups (1.6% in the SLND group vs 3.1% in the ALND group; P = .11), and there was no difference in DFS or OS.[21]
The results of ACOSOG Z0011 were criticized because the trial was considered underpowered, given the early study closure, and because it included only low-risk patients with favorable breast cancers. Nonetheless, the predefined statistical analysis was carried out. While no significant differences between the groups were noted, it is reassuring that the locoregional recurrence rates numerically favored the SLNB group, without any suggestion that the lack of significance could be attributed to the study being underpowered. And while the majority of enrolled patients were postmenopausal women with ER-positive tumors, that profile mirrors that of the breast cancer patient population in general. A subsequent prospective study performed at Memorial Sloan-Kettering Cancer Center, which enrolled 287 consecutive patients who met ACOSOG Z0011 eligibility criteria, demonstrated a very similar patient profile, with a median age of 58 years, 61% with T1 tumors, 90% with ER- and/or progesterone receptor (PR)-positive tumors, and 27% with H&E-detected micrometastatic axillary disease. Within this cohort of SLN-positive patients who met ACOSOG Z0011 criteria, completion ALND was avoided in 84% of women.[22] Because only 15% of women enrolled in ACOSOG Z0011 had ER- and PR-negative tumors, the applicability of the Z0011 results to women with triple-negative cancers was questioned. However, studies have shown that ER status is not a predictor of regional recurrence,[23,24] and other studies suggest that patients with triple-negative tumors are less likely to have axillary nodal involvement at all, and less likely than those with ER-positive tumors to have metastases to four or more axillary nodes.[25,26] While ACOSOG Z0011 raised concerns among surgeons and radiation oncologists that known nodal metastases might be left behind, it is important to remember that the randomized controlled trials that established the safety of SLNB alone in node-negative and low-volume disease (arbitrarily defined as ≤ 2 mm of metastasis) all show results nearly identical to those of Z0011. Compared wtih NSABP B-04,[1] which showed an axillary recurrence rate of 19% in women with larger tumors treated with mastectomy alone and no systemic therapy, in cohorts of low-to-moderate–risk breast cancer patients who are treated with whole-breast RT following lumpectomy and appropriate systemic therapy, the incidence of axillary recurrence, even in the undissected axilla, is extremely low and < 1% in all of the above-mentioned trials.[3,4,12,13] The ACOSOG Z0011 results are practice changing and are being incorporated into recommendations. NCCN guidelines now state that within a defined patient population (patients with a T1 or T2 tumor, 1 to 2 positive SLNs, and treatment with lumpectomy and whole-breast RT), one may consider no further axillary surgery.[10]
AMAROS
Similar to the ACOSOG Z0011 trial, the AMAROS (After Mapping of the Axilla: Radiotherapy or Surgery?) trial examined ways to decrease the morbidity of surgery in the modern multidisciplinary era by evaluating axillary RT as an alternative to ALND. AMAROS randomized women with clinically node-negative breast cancer, a tumor size between 0.5 and 3 cm, and a positive SLN to either completion ALND or axillary RT. Recently presented results include an axillary recurrence rate of 0.54% in the ALND group, and a rate of 1.03% in the group in which SLNB was followed with axillary RT, at median follow-up of 6.1 years. Given the low event rate, the noninferiority test was underpowered; still, no significant differences were observed.[27] A prior substudy of the first 2,000 women accrued to the trial provides detailed patient characteristics of this cohort; these included a median age of 57 years, 61% postmenopausal, and 74% with T1 tumors. Of patients in this initial cohort, 34% had a positive SLN, 63% had macrometastases, 25% had micrometastases, and 12% had ITCs.[28] While this study shows that SLNB plus axillary RT is an alternative to ALND, with equivalent outcomes and lower morbidity, it does not indicate that all patients with a positive SLN require axillary RT. Patient and tumor characteristics available from the AMAROS trial are very similar to the those in the ACOSOG Z0011 study, and both studies reported an approximately 1% rate of axillary recurrence in the experimental arm, questioning the actual benefit of axillary RT in addition to whole-breast RT for women treated with breast-conserving surgery. However, the AMAROS trial also included a group of women treated with mastectomy, and further evaluation of this cohort may provide interesting results in a population in which the omission of ALND has not been previously studied.
A potential concern regarding the omission of ALND in patients with positive SLNs is whether the additional pathologic information from the axillary contents would have an impact on adjuvant therapy recommendations. The factors associated with the administration of adjuvant chemotherapy were analyzed in the first 2,000 women enrolled in the AMAROS trial, and no differences based on type of axillary surgery were found in the number of patients treated with endocrine therapy, chemotherapy, or a combination of both. On multivariate analysis, the extent of nodal involvement was not significantly associated with the use of systemic therapy. Factors that were associated with the use of adjuvant systemic therapy included age, tumor grade, multifocality, and the size of the SLN metastasis (odds ratios for receiving chemotherapy: single ITC, 1; clusters of ITCs, 1.85; micrometastasis, 4.90; macrometastasis, 9.83; P = .0001), strongly suggesting that the information needed to make systemic therapy choices is available from analysis of the primary tumor and the sentinel node.[28]
MA.20
In contrast to ACOSOG Z0011 and AMAROS, which examined alternatives to ALND in patients with limited SLN involvement, the MA.20 trial examined the incremental benefit of adding nodal RT to ALND in a population similar to the populations included in ACOSOG Z0011 and AMAROS. Randomization was between RT with standard breast tangents-and breast tangents plus regional nodal radiation, including the supraclavicular, infraclavicular, and ipsilateral internal mammary chain. Axillary fields were added for women with ≥ 4 positive lymph nodes or < 10 lymph nodes removed in the ALND specimen. Inclusion criteria were either node-positive disease or node-negative disease with the following characteristics: tumors > 2 cm, < 10 axillary lymph nodes removed at the time of surgery, and at least 1 additional high-risk feature (grade 3 histology, ER-negative disease, or the presence of lymphovascular invasion).[29] The trial accrued 1,832 patients from 2000 to 2007 (median age, 53 years; 91% of patients received adjuvant chemotherapy; 71% received endocrine therapy). A median of 12 lymph nodes were removed at ALND. Eighty-five percent of patients had 1 to 3 positive lymph nodes, 5% had > 4 positive lymph nodes, and 10% were node-negative. At a median follow-up of 62 months, local recurrence rates were similar between the two groups, but there were fewer regional recurrence events in the nodal RT arm (21 events in the control group vs 4 events in the experimental group), with an absolute benefit in locoregional recurrence of 2.3% in the nodal RT group. The 5-year DFS was significantly improved with the addition of regional RT compared with whole-breast RT alone (89.7% vs 84.0%; P = .003). A trend toward improved 5-year OS was noted in the regional RT group (92.3% vs 90.7%; P = .07). Adverse outcomes were increased in those women treated with regional RT, including grade 2 or greater pneumonitis, lymphedema, and patient-reported poorer cosmetic outcomes.[30] Additional results from this trial are needed before conclusions can be drawn about the benefit of regional RT in this heterogeneous patient population.
It is difficult to compare these results with those of the ACOSOG Z0011 and AMAROS trials, since the inclusion criteria differ and the regional recurrence rate in the ALND arm is substantially higher in the MA.20 trial. The number of women with > 2 positive nodes or with gross nodal extracapsular extension, factors that were exclusion criteria in the ACOSOG Z0011 trial, is not available at this time. It is possible that for women with greater axillary disease burden, an ALND with a median number of 12 lymph nodes removed is not adequate axillary surgery, since this is far fewer than the number of lymph nodes removed in the ALND specimens from other randomized trials (median number of lymph nodes removed, 17 to 24).[3,20] Further analysis of the risk factors for regional recurrence within this patient population would be beneficial to help identify cohorts of high-risk women who might benefit from additional regional RT. As shown in the ACOSOG Z0011 and AMAROS trials, patients with lower-risk breast cancer have very low axillary recurrence rates, and additional regional treatment in this patient population is likely not needed, allowing the avoidance of ALND and regional RT morbidities.
Summary of Recommendations
While the NCCN provides locoregional management guidelines, as outlined, the area of axillary management is rapidly evolving; hence, guidelines are often out of date and may mislead as more recent research is integrated into clinical practice. At our institution, we have collectively adapted a number of strategies to manage the clinically node-negative axilla, as defined by physical examination (no routine axillary imaging is performed), in women undergoing initial surgical management; these strategies are outlined in Figure 1. SLNB is used to stage all clinically node-negative, non-T4 invasive breast cancers. SLNs are evaluated by H&E alone, without routine IHC staining. If the SLNs are negative or contain only ITCs, no additional axillary therapy is recommended. If micrometastases are identified with routine H&E staining, ALND is not performed, except in the uncommon circumstance in which the identification of macrometastases would change adjuvant therapy recommendations. For women undergoing mastectomy or those having a lumpectomy who do not meet ACOSOG Z0011 criteria, such as patients planning partial breast irradiation, SLNs are evaluated by frozen section at the time of surgery, and completion ALND is performed for macrometastatic lymph node disease. In the setting of breast conservation, for women who meet ACOSOG Z0011 criteria (node-negative by clinical examination, with T1 or T2 tumors, and for whom whole-breast radiation therapy without neoadjuvant chemotherapy is planned), SLNB is performed and no frozen section is obtained. If final pathology demonstrates one or two SLNs with macrometastases without gross extracapsular extension, no additional axillary surgery is recommended, and patients are treated with conventional radiation tangent fields. For women with ≥ 3 positive SLNs or grossly matted nodes, a completion ALND is performed. At this time, there is no consensus on the appropriate management of patients with microscopic extracapsular extension; this is under investigation. The appropriateness of axillary radiation vs completion ALND for low-volume axillary disease in women treated with breast-conserving therapy or mastectomy as in the AMAROS study will be evaluated after the publication of that study’s results. Similarly, determining the subset of patients who are best treated with ALND and nodal irradiation awaits the publication of the MA.20 trial results.
Figure 2 outlines our management recommendations for women with clinically node-positive disease. Ultrasound-guided fine-needle aspiration or core needle biopsy is performed to document the presence of metastatic disease. If the needle biopsy specimen is positive, management is with ALND. If the needle biopsy specimen is negative, SLNB is performed, with attention to the removal of all palpably abnormal lymph nodes, regardless of tracer uptake, since grossly positive nodes may not map due to lack of tracer uptake. The SLNB specimen is sent for frozen section and, if positive, a completion ALND is performed.
Clinically node-negative patients who present with abnormal preoperative axillary ultrasound performed prior to referral or with a positive needle biopsy performed for abnormal nodes seen on imaging are managed based on the number of abnormal lymph nodes identified. If < 3 abnormal lymph nodes are seen, then SLNB is performed without further preoperative workup. If ≥ 3 abnormal lymph nodes are identified, then ultrasound-guided needle biopsy is performed and the results determine surgical management. All of the above recommendations apply only to patients undergoing initial surgery. Axillary management in women treated with neoadjuvant chemotherapy is an active area of investigation that is beyond the scope of this review.
Conclusion
The SLNB procedure has expanded to provide accurate staging information and adequate regional control in node-negative patients and those with a limited number of positive lymph nodes. What has been learned from the evolution of the SLNB trials is that arbitrary cut-offs between ITCs, micrometastatic nodal disease, and macrometastatic nodal disease are not adequate for determining the appropriate extent of axillary surgery. Rather, a spectrum of tumor and nodal characteristics are emerging that can determine the most appropriate regional therapy for individual patients. Because rates of regional recurrence continue to decrease with increased utilization of adjuvant therapy, and because the potential morbidity of ALND and regional RT is substantial, further research is needed to identify specific cohorts of patients who actually benefit from more aggressive locoregional treatments. As adjuvant breast cancer therapy progresses, the impact of axillary surgery for regional control will likely continue to lessen, and the significance of the anatomic lymph node disease burden will be trumped by tumor biology.
Financial Disclosure:The authors have no significant financial interest in or other relationship with the manufacturer of any product or provider of any service mentioned in this article.
References:
1. Fisher B, Jeong JH, Anderson S, et al. Twenty-five-year follow-up of a randomized trial comparing radical mastectomy, total mastectomy, and total mastectomy followed by irradiation. N Engl J Med. 2002;347:567-75.
2. Anderson SJ, Wapnir I, Dignam JJ, et al. Prognosis after ipsilateral breast tumor recurrence and locoregional recurrences in patients treated by breast-conserving therapy in five National Surgical Adjuvant Breast and Bowel Project protocols of node-negative breast cancer. J Clin Oncol. 2009;27:2466-73.
3. Veronesi U, Paganelli G, Viale G, et al. Sentinel-lymph-node biopsy as a staging procedure in breast cancer: update of a randomised controlled study. Lancet Oncol. 2006;7:983-90.
4. Krag DN, Anderson SJ, Julian TB, et al. Sentinel-lymph-node resection compared with conventional axillary-lymph-node dissection in clinically node-negative patients with breast cancer: overall survival findings from the NSABP B-32 randomised phase 3 trial. Lancet Oncol. 2010;11:927-33.
5. Krag DN, Anderson SJ, Julian TB, et al. Technical outcomes of sentinel-lymph-node resection and conventional axillary-lymph-node dissection in patients with clinically node-negative breast cancer: results from the NSABP B-32 randomised phase III trial. Lancet Oncol. 2007;8:881-8.
6. van der Ploeg IM, Nieweg OE, van Rijk MC, et al. Axillary recurrence after a tumour-negative sentinel node biopsy in breast cancer patients: a systematic review and meta-analysis of the literature. Eur J Surg Oncol. 2008;34:1277-84.
7. Kim T, Giuliano AE, Lyman GH. Lymphatic mapping and sentinel lymph node biopsy in early-stage breast carcinoma: a metaanalysis. Cancer. 2006;106:4-16.
8. Mansel RE, Fallowfield L, Kissin M, et al. Randomized multicenter trial of sentinel node biopsy versus standard axillary treatment in operable breast cancer: the ALMANAC Trial. J Natl Cancer Inst. 2006;98:599-609.
9. Veronesi U, Paganelli G, Viale G, et al. A randomized comparison of sentinel-node biopsy with routine axillary dissection in breast cancer. N Engl J Med. 2003;349:546-53.
10. National Comprehensive Cancer Network (NCCN). NCCN Clinical Practice Guidelines in Oncology Breast Cancer. Version 1.2014. 2014. Available from: https://www.nccn.org/professionals/physician_gls/pdf/breast.pdf Accessed April 11, 2014.
11. Giuliano AE, Dale PS, Turner RR, et al. Improved axillary staging of breast cancer with sentinel lymphadenectomy. Ann Surg. 1995;222:394-9; discussion 99-401.
12. Galimberti V, Cole BF, Zurrida S, et al. Axillary dissection versus no axillary dissection in patients with sentinel-node micrometastases (IBCSG 23-01): a phase 3 randomised controlled trial. Lancet Oncol. 2013;14:297-305.
13. Sola M, Alberro JA, Fraile M, et al. Complete axillary lymph node dissection versus clinical follow-up in breast cancer patients with sentinel node micrometastasis: final results from the multicenter clinical trial AATRM 048/13/2000. Ann Surg Oncol. 2013;20:120-7.
14. Giuliano AE, Hawes D, Ballman KV, et al. Association of occult metastases in sentinel lymph nodes and bone marrow with survival among women with early-stage invasive breast cancer. JAMA. 2011;306:385-93.
15. Weaver DL, Ashikaga T, Krag DN, et al. Effect of occult metastases on survival in node-negative breast cancer. N Engl J Med. 2011;364:412-21.
16. Julian TB, Anderson SJ, Krag DN, et al. 10-year follow-up results of NSABP B-32, a randomized phase III clinical trial to compare sentinel node resection to conventional axillary dissection in clinically node-negative breast cancer patients. J Clin Oncol. 2013;31:
17. Bilimoria KY, Bentrem DJ, Hansen NM, et al. Comparison of sentinel lymph node biopsy alone and completion axillary lymph node dissection for node-positive breast cancer. J Clin Oncol. 2009;27:2946-53.
18. Cserni G, Gregori D, Merletti F, et al. Meta-analysis of non-sentinel node metastases associated with micrometastatic sentinel nodes in breast cancer. Br J Surg. 2004;91:1245-52.
19. van Deurzen CH, de Boer M, Monninkhof EM, et al. Non-sentinel lymph node metastases associated with isolated breast cancer cells in the sentinel node. J Natl Cancer Inst. 2008;100:1574-80.
20. Giuliano AE, McCall L, Beitsch P, et al. Locoregional recurrence after sentinel lymph node dissection with or without axillary dissection in patients with sentinel lymph node metastases: the American College of Surgeons Oncology Group Z0011 randomized trial. Ann Surg. 2010;252:426-32; discussion 32-3.
21. Giuliano AE, Hunt KK, Ballman KV, et al. Axillary dissection vs no axillary dissection in women with invasive breast cancer and sentinel node metastasis: a randomized clinical trial. Jama. 2011;305:569-75.
22. Dengel LT, Van Zee KJ, King TA, et al. Axillary dissection can be avoided in the majority of clinically node-negative patients undergoing breast-conserving therapy. Ann Surg Oncol. 2014;21:22-7.
23. Yates L, Kirby A, Crichton S, et al. Risk factors for regional nodal relapse in breast cancer patients with one to three positive axillary nodes. Int J Radiat Oncol Biol Phys. 2012;82:2093-103.
24. Grills IS, Kestin LL, Goldstein N, et al. Risk factors for regional nodal failure after breast-conserving therapy: regional nodal irradiation reduces rate of axillary failure in patients with four or more positive lymph nodes. Int J Radiat Oncol Biol Phys. 2003;56:658-70.
25. Ugras S, Stempel M, Patil S, Morrow M. Breast cancer molecular subtype predicts lymphovascular invasion (LVI) and lymph node involvement. Presented at the 2014 Society of Surgical Oncology Annual Cancer Symposium; March 12–15, 2014; Phoenix, AZ.
26. Wiechmann L, Sampson M, Stempel M, et al. Presenting features of breast cancer differ by molecular subtype. Ann Surg Oncol. 2009;16:2705-10.
27. Rutgers EJ, Donker M, Straver ME, et al. Radiotherapy or surgery of the axilla after a positive sentinel node in breast cancer patients: Final analysis of the EORTC AMAROS trial (10981/22023). J Clin Oncol. 2013;31(suppl):abstr LBA1001.
28. Straver ME, Meijnen P, van Tienhoven G, et al. Role of axillary clearance after a tumor-positive sentinel node in the administration of adjuvant therapy in early breast cancer. J Clin Oncol. 2010;28:731-7.
29. Olivotto IA, Chua B, Elliott EA, et al. A clinical trial of breast radiation therapy versus breast plus regional radiation therapy in early-stage breast cancer: the MA20 trial. Clin Breast Cancer. 2003;4:361-3.
30. Whelan TJ, Olivotto I, Ackerman I, et al. NCIC-CTG MA.20: An intergroup trial of regional nodal irradiation in early breast cancer. J Clin Oncol. 2011;29:abstr LBA1003.