Markers May Help Select Patients for Adjuvant Therapy
This special “annual highlights” supplement to Oncology News International is acompilation of some of the major advances in the management of gastrointestinalcancers during 2003–2004, as reported in ONI. Guest editor Dr. James L. Abbruzzesecomments on the reports included herein and discusses advances in the clinicalmanagement of GI cancers, with a focus on developments in targeted therapy, newcombinations, adjuvant therapy, and what to watch for in 2004.
PALM BEACH, Florida-Integratingmolecular biology with patientcare will require testing methodsthat are feasible in routine care, reproducible,and manufactured with goodquality assurance, according to Al B.Benson III, MD.Early work in colorectal cancer suggeststhat such tests can be developed,that they will improve the efficiency ofclinical trials, and that they will helpclinicians select regimens most likelyto benefit the individual patient, Dr.Benson said at the First InternationalColorectal Cancer Congress. He is directorof clinical investigations, NorthwesternUniversity Robert H. LurieComprehensive Cancer Center,Chicago."At present, eligibility criteria forclinical trials represent a generic populationthat includes a high percentageof patients who will receive nobenefit from the study treatment," Dr.Benson said. "Targeting markers, suchas those for epidermal growth factorreceptor (EGFR) or vascular endothelial growth factor (VEGF), may helpavoid giving patients a test drug fromwhich they can expect only toxicity."
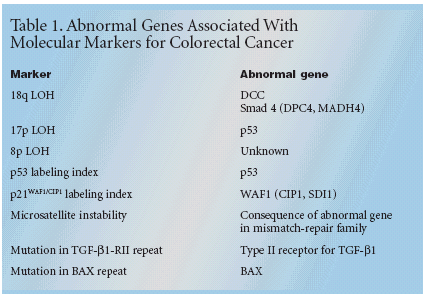
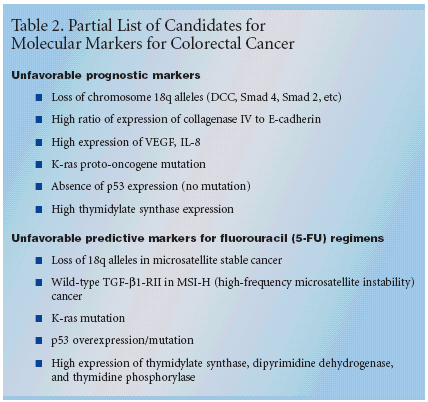
For a marker to become clinicallyuseful, it must have significant andindependent value, be validated byclinical testing, be feasible for routineuse, be reproducible, and be widelyavailable with rigorous quality controls,Dr. Benson noted. "Furthermore,we should demonstrate that agiven result will offer benefit to theindividual patient," he said.The list of candidates for clinicallyuseful markers in colorectal cancer islong (see Tables 1 and 2). Some ofthese will be markers for unfavorableprognosis, suggesting that the patientis a candidate for particularly aggressivetreatment. Others are predictorsof response to a specific drug such asfluorouracil (5-FU). "These includeloss of the 18q allele in microsatellitestable cancer, mutations of the genefor type II receptor for TGF-β1 (TGF-β1-RII) in cancers with microsatelliteinstability (MSI), and high expression of thymidylate synthase," Dr.Benson said.
One challenge for clinical investigatorsis how to help patients understandwhy taking tissue samples formarker analysis is important. "Manytrials require additional patient approvalfor tissue acquisition, and recentprivacy laws in the United Stateswith their accompanying regulatoryprocesses may make it much moredifficult to obtain tissue and to establishtissue banks," he said.Tissue acquisition increases the costof conducting clinical trials, and newfunding sources will be required toestablish tissue banks and develop usabletests for tumor markers. He saidthat there is a tension between industry'spush for rapid drug developmentand researchers' need for biologicallydriven trials.Multidisciplinarycollaborationrequired"This will require multidisciplinarycollaboration. In the past,most trials did not include fundingfor the pathologists and surgeons, whowill be critical for obtaining and evaluatingtissue for our biological analyses,"Dr. Benson said.Small uncontrolled or retrospectivecorrelational trials may not providedefinitive answers about the predictivepower of a molecular marker,he said. "We must resist the temptationto use the growing number ofcommercial laboratories that markettests for molecular and other markers,"Dr. Benson stressed.Trial DataData from ECOG patients in intergrouptrials of stage III colon cancerpatients showed that retention of the18q allele, TGF-β1-RII, and MSI witha TGF-β1-RII mutation were associatedwith favorable outcome after 5-FU. Five-year survival was 74% in patientswho had the 18q allele vs 50% inthose without the allele, and 74% inthose who had MSI but also had theTGF-β mutation vs 46% in those withMSI but no such mutation.Dr. Benson said that clinical trialE5202, which is now on the drawingboard for patients with stage II coloncancer, would be the first to prospectivelyevaluate presence of the 18q alleleand MSI status in tissue samplesobtained immediately after surgery.High-risk and low-risk profiles will bedetermined according to whether thepatient has microsatellite stability buthas lost the 18q allele (high risk) or hasMSI without loss of the 18q allele(low risk).Low-risk patients will be assignedto observation, and Dr. Benson estimatedthat survival of low-risk patientsshould approach 90%. Highriskpatients will be randomized to5-FU/ leucovorin or to an experimentalarm that will include a targetedtherapy yet to be determined.