Melanoma in the Older Person
Melanoma incidence and mortality continue to rise unabated in older individuals. Early clinical detection should take into account the different subtypes.
Melanoma accounts for the majority of skin cancer deaths worldwide and has dramatically increased in incidence over the past halfcentury. Despite recent trends showing improved survival, and stabilization of incidence rates in younger Americans, melanoma incidence and mortality continue to rise unabated in older individuals, particularly in men over age 65. Efforts at early clinical detection of melanoma in older individuals should take into account the differences in melanoma subtypes in older individuals, potentially reduced access to medical specialists in this population, as well as comorbidities that may affect ability to undergo treatment for advanced disease. Secondary melanoma prevention should be focused on targeted education to older men and their spouses for early detection and reduction of mortality in this extremely high-risk group.
Over 55,000 white adults in the United States are expected to develop invasive cutaneous malignant melanoma in 2004, and 7,900 patients will die from metastatic disease within the next year.[1] The estimated lifetime risk for melanoma is currently 1 out of 68 Americans, and this number is expected to rise to 1 in 50 by the year 2010.[2] Risk factors including greater occupational and recreational sun exposure have resulted in an increased incidence of melanoma over the past 50 years, although earlier detection and treatment of thinner lesions have contributed to improved patient survival, particularly in younger individuals.[3,4]
The most striking differences in melanoma incidence and mortality occur in individuals over age 65, although modest differences in age-specific incidence and mortality are notable in those over age 50. Older individuals are both more likely to acquire and to die from melanoma, and the elderly should therefore be a primary target for secondary melanoma prevention, ie, early detection and screening to reduce melanoma mortality. Treatment options in the elderly may also be limited due to decreased inability to tolerate medication side effects or toxicity, comorbid medical conditions, increased likelihood of drug interactions, and potential exclusion from clinical trials based on age eligibility criteria.
Melanoma Incidence and Mortality in the Elderly
Early clinical detection of malignant melanoma has the greatest impact on prolonged survival and potential eradication of disease. Earlier diagnosis and treatment of thinner cutaneous melanomas has contributed to a decreased case-based fatality rate in the United States over the past 50 years, despite an overall increase in melanoma incidence.[5,6] Risk factors for development of melanoma include fair skin type, strong family history of melanoma, significant sun exposure (particularly blistering sunburns), the presence of numerous and/or clinically atypical moles, and importantly, older age.
The reasons for the increasing melanoma incidence have yet to be fully defined; it remains controversial whether increasing melanoma incidence is real or simply reflects improved detection of earlier, thinner lesions. For example, in a recent analysis of the Surveillance, Epidemiology, and End Results (SEER) program from 1973 to 1997, the incidence of thin melanomas (< 1 mm depth) increased significantly in all age groups except for men under age 40. Most importantly, this study showed that rates of thick melanomas (≥ 4 mm) have increased significantly only in males aged 60 years and older.[7]
FIGURE 1

Mortality and Incidence
Additional analyses of the SEER mortality (1969-1999) and incidence (1973-1999) databases has yielded notable results regarding the effect of age on melanoma risk and outcome. Analyses were age-adjusted and rates were expressed as deaths per 100,000 and standardized to the 2000 US population.[ 8,9] Data were analyzed separately for white men and women in the following age groups, 20-44, 45-64, and 65+ years. Trends were analyzed separately for each of the six sex/age groups and overall. All age-specific trends and differences between men and women were significant at P < .01 (Figure 1).
Overall, melanoma mortality rates rose from 2.0 per 100,000 in 1969 to 3.0 in 1999, but with striking differences by age and sex. Mortality rates rose 19% in middle-aged women (45- 64 years, 2.6 to 3.1 per 100,000) and 66% in middle-aged men. Most alarming, mortality rates increased 157% in older men (7.5 to 19.3 per 100,000), more than threefold greater than the increase for older women. Incidence data generally paralleled mortality data for the same period, revealing a threefold increase among middle-aged men (13.5 to 40.5 per 100,000) and a nearly fivefold increase in older men (18.8 to 91.9 per 100,000) but less than a doubling for younger men (6.8 to 11.6 per 100,000). Incidence also increased for women, with the same pattern of greater increases in older age groups but less strikingly than in men.
This analysis also yielded important differences in tumor thickness and histology by gender and age. Median tumor- thickness measurements were as follows: 0.54 mm for younger women, 0.64 mm for older women, 0.64 mm for younger men, and 0.67 mm for older men. For all histologic subtypes other than lentigo maligna melanoma, men 50 years of age and older (compared with other age/sex groups) were most likely to be diagnosed with thick (≥ 2.0 mm) tumors. In contrast, younger women had fewer thick melanomas in all histologic subtypes.
Among men age 50 and above, 19% of all melanomas were ≥ 2 mm, more than double the 8% rate among younger women. Women ≥ 50 years old had thicker nodular melanomas than women under age 50 (median: 2.29 vs 1.79 mm). Likewise, men ≥ age 50 had thicker nodular melanomas compared to women less than age 50 (median: 2.39 vs 2.04 mm). Among men and women ≥ age 50, nodular melanoma greater than 2 mm comprised 60% and 57% of all nodular melanomas vs 57% and 45% for men and women less than age 50.[10] These age- and gender-based differences in tumor depth and histogenetic subtype emphasize the need for early detection efforts aimed at the elderly population and older men in particular.
Precursor Lesions
Malignant melanoma may arise de novo or from a precursor melanocytic nevus. A changing nevus is the most important risk factor for melanoma, and variation in size, shape, or color of the preexisting nevus, or onset of bleeding, pain, or pruritus within a mole is noted by over 80% of melanoma patients at the time of diagnosis.[ 11] Precursor lesions include congenital nevi (particularly the giant or "bathing trunk" type), common nevi, clinically atypical (or dysplastic) nevi, and melanoma in situ (lentigo maligna, superficial spreading melanoma in situ, and acral lentiginous melanoma in situ).
Although most patients with primary melanoma report preexisting pigmented lesions, the actual percentage of melanomas confirmed histologically to arise from a preexisting nevus is unclear. Most studies suggest that only about one-third of melanomas arise from a precursor nevus (common, dysplastic, or congenital), although the percentage may actually be higher (≥ 50%) due to possible histologic obliteration of the underlying nevus by deeper melanomas.[12-14]
Elderly patients tend to have fewer nevi in association with their melanomas, likely related to differences in melanoma subtype prevalence, ie, fewer superficial spreading melanomas relative to other histogenetic types in older individuals.[15,16] It has also been suggested that more melanomas arise de novo with increasing age. However, the risk for tranformation of a single nevus into melanoma may be greater with age in part due to declining nevus counts in the older population.[17] Regardless of whether a melanoma arises de novo or from a preexisting nevus, patients and practitioners will often recognize it as a "changing mole," and certain clinical features may aid in prompt and accurate diagnosis.
Physician and patient education regarding the warning signs of early melanoma has been promoted in the United States with the use of the "ABCD" criteria for a changing mole, which includes asymmetry ("A"), border- notching ("B"), color variegation ("C") with black, brown, red, blue, or white hues, and diameter ("D") greater than 6 mm (commonly referred to as greater than the size of a pencil eraser) or any noted growth of a preexisting pigmented lesion.[18] Lesions exhibiting these features should be considered potential melanomas, although severely dysplastic nevi may be difficult to distinguish clinically.
Primary Cutaneous Melanoma
Primary cutaneous melanoma may occur anywhere on the body, although it is most commonly diagnosed on the lower extremities and back in women, and the trunk in men.[19] Certain melanoma subtypes, such as lentigo maligna melanoma and acral lentiginous melanoma, occur in characteristic locations as discussed below. In addition to the ABCD criteria, surface features such as elevation and ulceration may be useful in predicting whether melanoma is early or advanced.
Lateral growth of a pigmented macule is believed to correspond to the in situ or microinvasive (upper papillary dermal) component, whereas the development of raised or indurated areas within the clinical lesion suggests progression to vertical growth in the dermis, subcutaneous fat, or deeper. Progression from radial (or horizontal) growth to vertical growth is believed to give melanoma the potential to metastasize.[20] Ulceration is typically seen in melanomas in the vertical growth phase and is a clinical and histologic indicator of worse prognosis.[21]
Histogenetic Subtypes
There are four major histogenetic subtypes (or growth patterns) of primary cutaneous melanoma: superficial spreading melanoma, nodular melanoma, acral lentiginous melanoma, and lentigo maligna melanoma. Desmoplastic melanoma is a less common but important melanoma subtype to recognize, given its predilection for older individuals and clinical features similar to nonmelanoma (keratinocytic) skin cancer. Distinction among subtypes is largely based on anatomic site, and it remains controversial as to whether melanoma subtype affects overall prognosis. With the exception of nodular melanoma, all growth pat- terns are characterized by a preceding in situ (radial growth) phase, which is biologically benign but morphologically malignant. This indolent phase of intraepithelial growth lacks the biologic potential to metastasize and may last from months to years before invasion occurs. As such, melanoma in situ is completely cured following excisional surgery.[20,22]
FIGURE 2
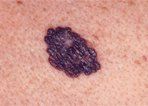
Superficial Spreading Melanoma
• Superficial Spreading Melanoma-Superficial spreading melanoma is the most common subtype of melanoma, accounting for about 70% of all cases, particularly between the ages of 30 and 50.[23] In the elderly population, superficial spreading melanoma is estimated to comprise 40% to 50% of cases.[24] It occurs most frequently on the upper back of men and women as well as the lower extremities of women. The clinical lesion typically shows irregular, asymmetric borders with color variegation (eg, black, blue, or pink), and size generally greater than 6 to 8 mm (Figure 2). Hypopigmentation (tan, white, or gray discoloration) is not uncommon and corresponds to areas of tumor regression or pigment incontinence (melanin deposition in the dermis).
The clinical differential diagnosis includes both benign and malignant neoplasms. The most common melanoma simulants are seborrheic keratoses (benign tan to dark brown keratinocytic proliferations) and traumatized nevi, which may present as a hemorrhagic or "bleeding mole." In addition, a nevus showing severe atypia may be clinically indistinguishable from a melanoma. In this case, a history of gradual or recent change in a preexisting mole may help to differentiate early melanoma from a longstanding dysplastic nevus, although histopathologic examination should be performed if there is any doubt. Pigmented basal cell carcinoma may also be confused with superficial spreading or nodular melanoma.
FIGURE 3
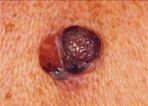
Nodular Melanoma With Ulceration
• Nodular Melanoma-Nodular melanoma is the second most common subtype of melanoma, accounting for 15% to 30% of all types, and is more common in men than women.[ 23] The median age of diagnosis is 53 years; however, thicker nodular melanomas are associated with older age.[10] Like superficial spreading melanoma, the legs and trunk are the most frequent sites of involvement. However, rapid growth over weeks to months is a hallmark of nodular melanoma and corresponds to its lack of a preceding in situ (or radial growth) phase.
Clinically, the lesion presents as a raised, dark brown to black papule or nodule, and ulceration and bleeding are common (Figure 3). Nodular melanoma may resemble a pyogenic granuloma, traumatized nevus, or seborrheic keratosis, although amelanotic (nonpigmented) variants may mimic basal cell carcinoma, squamous cell carcinoma, or benign fibrohistiocytic tumors. Nodular melanoma is often associated with a worse prognosis because it may not exhibit the typical ABCD characteristics of melanoma, thus eluding early detection and often demonstrating greater tumor depth at the time of diagnosis. However, despite its seemingly more aggressive clinical behavior, nodular melanoma has a prognosis similar to superficial spreading melanoma when matched for tumor thickness.[25]
Recent analysis of melanoma subtypes has demonstrated that the nodular subtype accounts for the vast majority of thick tumors at the time of diagnosis.[26,27] Likewise, patients with thick nodular melanoma (> 2-mm depth) are significantly older at diagnosis compared to patients with superficial spreading melanoma, with one study showing a mean age of 63 vs 59, respectively.[26] Since nodular melanoma tends to elude early detection, public educational efforts focused at symptoms, such as increase in lesion diameter or height and onset of bleeding, may be more useful than traditional signs of thin melanomas, such as change in color.[ 27] In fact, a significant proportion of nodular melanomas are amelanotic, and thus the "color" criterion typically used for detection of suspicious change in pigmented lesion morphology may not apply.
FIGURE 4
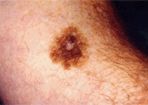
Lentigo Maligna
• Lentigo Maligna Melanoma-Lentigo maligna melanoma accounts for 4% to 15% of cutaneous melanomas and is typically located on the head, neck, and arms (sun-damaged skin) of elderly, fair-skinned individuals (mean age: 65).[23] However, recent characterization of melanoma subtype incidence has suggested increasing rates of both in situ and invasive lentigo maligna subtypes, particularly in individuals greater than age 50.[28]
FIGURE 5
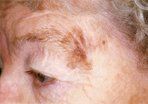
Lentigo Maligna Melanoma
Like nonmelanoma skin cancer, lentigo maligna melanoma is linked to cumulative, rather than intermittent, sun exposure. The face is the most common site of involvement, particularly the nose and cheeks. The precursor in situ lesion, lentigo maligna, is usually present for over 5 to 20 years and often attains large size (> 3-cm diameter) before progression to lentigo maligna melanoma occurs. Lentigo maligna appears as a tan to brown macule or patch with variation in pigment or areas of regression that appear hypopigmented clinically (Figure 4).
Only 5% to 8% of lentigo malignas are estimated to evolve to invasive melanoma, and this event is characterized by nodular development within the flat precursor lesion (Figure 5).[29] The clinical differential diagnosis includes superficial spreading melanoma and benign solar lentigines that are typically smaller, evenly pigmented, and flat.
FIGURE 6
Acral Lentiginous Melanoma
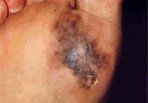
• Acral Lentiginous Melanoma-Acral lentiginous melanoma is the least common subtype, representing only 2% to 8% of melanoma in whites, although it accounts for 29% to 72% of melanoma in dark-complexioned individuals (African-Americans, Asians, and Hispanics).[23,30] It typically occurs on the palms or soles or beneath the nail plate (subungual variant). A small percentage of superficial spreading and nodular melanoma may also be located acrally.[31] Patients are generally middle-aged to elderly, with an average onset in the sixth decade. Irregular pigmentation, large size (≥ 3 cm diameter), and plantar location are characteristic features of acral lentiginous melanoma (Figure 6).
FIGURE 7
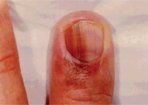
Hutchinson's Sign
Subungual melanoma occurs most commonly on the great toe or thumb and is characterized by the rapid onset of diffuse nail discoloration or a longitudinal pigmented band within the nail plate. The additional presence of pigmentation extending into the proximal or lateral nail folds (Hutchinson's sign) strongly suggests subungual melanoma and warrants biopsy of the nail matrix, from which these melanomas arise (Figure 7). Subungual melanoma may be confused with a benign junctional nevus, pyogenic granuloma, infectious process (bacterial or fungal), or subungual hematoma. If subungual hematoma is suspected, a history of trauma should be elicited and the lesion followed to ensure resolution with continued growth of the nail plate.
Rare Melanoma Variants
Unusual subtypes of primary melanoma include desmoplastic/neurotropic melanoma, mucosal (lentiginous) melanoma, malignant blue nevus, melanoma arising in giant congenital nevus, and melanoma of soft parts (clear cell sarcoma). Together, these variants account for less than 5% of primary melanomas.
Desmoplastic melanoma may occur in association with macular, lentigo maligna-type pigmentation, or present de novo as a firm, amelanotic nodule or scar (Figure 8). Desmoplastic melanoma occurs predominantly on sun-exposed areas of the head and neck at a mean age between 60 and 65 years.[32,33] Lack of pigmentation and clinical features more suggestive of keratinocytic skin cancer (basal cell and squamous cell carcinoma) may result in delay in detection and thicker tumors at diagnosis.
Age as a Prognostic Factor
FIGURE 8
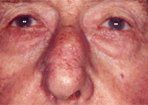
Desmoplastic Melanoma
Differences in disease-free and overall survival have been studied with regard to patient age. Older patients (> 65) tend to have thicker melanomas at the time of diagnosis and a greater percentage of ulcerated melanomas compared to younger patients-factors that adversely affect both recurrence and mortality rates.[34,35] The issue of whether age alone directly correlates with worse survival has been debated over the past several decades. Several multivariate analyses in the late 1970s and early 1980s assessed the independent prognostic value of multiple histopathologic variables (tumor thickness, ulceration, level of invasion, growth pattern, etc) and clinical prognostic factors (gender, age, tumor location) with regard to survival; these studies showed no direct effect of age on survival in patients with cutaneous melanoma.[36-39] However, more recent studies have suggested otherwise. In a stepwise regression analysis of 442 patients with cutaneous melanoma, Austin et al treated age as a continuous variable and showed that increasing age and Breslow thickness were the only significant predictors of disease-free survival.[ 34] Furthermore, inclusion of Clark level, ulceration, sex, and primary site did not add to the prognostic model.
Likewise, the worldwide melanoma database established in 1998 by the American Joint Committee on Cancer (AJCC) Melanoma Staging Committee yielded important results regarding the effect of patient age as an independent prognostic factor on melanoma-specific survival. The AJCC melanoma data set comprises the largest international database of primary determinants of tumor-nodemetastasis (TNM) categories on melanoma outcome. In a multivariate analysis of 13,581 patients with localized cutaneous melanoma, age followed thickness and ulceration as the third most important determinant of prognosis.[40] Patient age was also statistically significant in the AJCC Cox regression analysis of 4,750 clinically node-negative melanoma patients who underwent pathologic staging of regional lymph nodes after sentinel or elective lymphadenectomy. Thus, age appears to remain an important clinical prognostic factor in patients with and without regional nodal metastasis.
Reasons for differences in prognosis in older patients have been attributed to a diminished immune response with increased age,[41,42] changes in host immune biology,[15] decreased ability to repair DNA in sun-damaged melanocytes,[42] undertreatment with increasing age (narrower surgical margins, fewer staging procedures due to other medical conditions),[34] difficulty with skin self-examination due to failing eyesight or poor health, unaffordable or inaccessible adequate medical care, and living situations that may involve a lack of spouse or family to assist in health-care maintenance and early detection.[42] Differences in the reporting of signs and symptoms of melanoma in older vs younger populations have also been studied in an attempt to account for the increased proportion of thicker tumors in older patients.
In a study of 1,250 hospital- and population-based cases by Christos et al, older patients (≥ age 50) were less likely to report itching and change in elevation or color of their lesions, whereas ulceration was reported more frequently.[43] Ulceration and bleeding are characteristic signs of advanced melanoma, correlating with increased tumor thickness, delay in seeking medical attention, and a higher frequency of the nodular subtype in older individuals.[44-47] Self-detection practices in the elderly may be affected by decreased personal knowledge of signs and symptoms of melanoma and other behavioral factors. Studies have shown that older individuals have difficulty in discriminating early changes of melanoma in pigmented lesion photographs and decreased ability to recognize clinical changes of melanoma compared to younger individuals.[43,45,47]
Effect of Age on Melanoma Treatment
With increasing age, there is an accumulation of medical comorbidity that may limit therapy with antineoplastic agents, and particularly with the biologic agents known as cytokines and interferons. Many of the original trials of biologic antitumor agents in melanoma excluded patients who were older than 70 years or of diminished performance status. The objective toxicities of fever and capillary leak syndrome (for interleukin [IL]-2, Proleukin) have precluded treatment with patients who have underlying lung dysfunction and diminished diffusing capacity of the lung for carbon monoxide (DLCO), or limitations of cardiac function with congestive failure or angina.
The principal limitation of biologicals is their induction of a flu-like syndrome that can be particularly insidious in the elderly, who may have underlying organic syndromes or live alone, thereby escaping the day-to-day surveillance that younger patients experience in the course of work and home life. The recognition of inhibitory effects upon specific cytochrome p450 enzymes provides potential insight to drug combinations that may be prone to cause excessive toxicity with analgesics, opiates, and bronchodilators.[ 48] Recent recognition of the importance of aggressive supportive care to enable optimal therapy has led to recommendations that are pertinent for all patients but critical for the safe and effective treatment of the elderly.[49]
Secondary Prevention Efforts
Early Detection of Melanoma
The mean age at which melanoma is diagnosed is 53, with a predominance of new cases occurring in older individuals and particularly in men > 65. As discussed, older men have the highest melanoma risk in the United States and should be the targets of national screening efforts as well as professional and patient education campaigns directed toward earlier detection. Early detection of melanoma is associated with thinner tumors, which have a better prognosis.[20] However, early detection efforts in the elderly may be hampered by reduced access to medical specialists and changes in health insurance coverage. For instance, health providers may be reluctant to add Medicare patients to their practices due to lower reimbursement rates, and elderly patients may have increased difficulty obtaining both routine and specialized medical services.
Public awareness of the dangers of excessive sun exposure has increased due to the combined efforts of the American Academy of Dermatology (AAD), American Cancer Society, The Skin Cancer Foundation, and the American Academy of Pediatrics, which advocate primary prevention, especially in younger individuals, through avoidance of excessive sun exposure and sun protective measures. Skin cancer screenings have also enhanced early detection of melanomas nationwide.
Screening
Free skin cancer screenings have been endorsed by the AAD and conducted by volunteer dermatologists since 1985. Over 1.3 million individuals have been screened, and 122,000 suspicious lesions have been detected as of 2003, including approximately 14,400 suspected melanomas.[50] However, the value of skin cancer screening has come under scrutiny, in part due to the lack of postscreening outcome data to validate the practice of screening. Likewise, no randomized trials or case-control studies have addressed whether early detection via screening is effective in reducing mortality or morbidity from skin cancer. As a result, the third US Preventive Services Task Force (USPSTF) concluded that there is insufficient evidence to recommend for or against routine screening for skin cancer for the early detection of cutaneous melanoma, basal cell carcinoma, and squamous cell carcinoma. However, the USPSTF did call for studies "to help the clinician identify patients, especially the elderly, at high risk for melanoma."[51]
Furthermore, in 2000, the Institute of Medicine reached similar conclusions regarding general screening recommendations but conceded that "clinicians and patients should continue to be alert to the common signs of skin cancer-with a particular emphasis on older white males and on melanoma."[52] The report concluded that the major challenge related to the Medicare population is reaching the group at highest risk of death from skin cancer, specifically older fairskinned men.[52]
Routine individual or mass screening has been advocated by both the AAD and the American Cancer Society.[ 53] Prescreening advertising that targets high-risk individuals, such as those with fair skin, tendency to sunburn, increased mole count and/or dysplastic nevi, and family history of melanoma, has been shown to enhance community-based screenings, and a selective referral policy may be more useful when applied to the mass screening setting.[54-56] Likewise, a recent AAD-sponsored study suggested that the yield of mass screening for melanoma would be improved by targeting middle-aged and older men, with the greatest utility in men 50 years of age or older, and particularly in those with a history of "changing mole" or skin type I/II.[57]
Indeed, among all screenees, the highest yield of melanoma was found among those who were aged 50 years or older, male, had a changing mole, or had skin type I and II (fair complexion, tendency to sunburn). The overall yield of melanoma (expressed as the number of confirmed cases per 1,000 screenings) was 1.50 (363/ 242,374). The yield among men aged ≥ 50 years was 2.63, a factor of 1.8 greater than among men younger than age 50, 2.8 times greater than among women < 50 years, and 2.4 times greater than among women ≥ 50 years. Among middle-aged and older men, the yield of confirmed melanoma was even higher if they reported a changing mole (4.60/1,000) or skin type I/II (3.80/1,000). When both risk factors were present, the yield was 6.63 per 1,000 screenings.[57] Thus, middleaged and older men accounted for a disproportionately high number of detected melanomas, while representing only a small fraction of total screened individuals.
This suggests that in order to optimize benefit from mass skin cancer screening and public education, publicity campaigns should expand outreach to men aged 50 years and above. Efforts might center on workplaces and recreational activities frequented by men in this age group. Marketing strategies might also include specially crafted messages to middle-aged and older men as well as their spouses or partners.
Conclusions
Compared to colorectal, prostate, and breast cancer, melanoma is the only early-detectable cancer for which death rates are rising, but the number of screened individuals has changed very little or even diminished over the past decade.[58] Melanoma control programs should be directed to reaching the high-risk, unscreened population. Recent incidence and mortality data suggest the need to target older men in particular for increased melanoma awareness through public and professional education campaigns and for early detection through health-care provider or community- based skin cancer screening. Further research on both the behavioral and biologic fronts must work in tandem to elucidate the causes for the rising incidence and mortality of melanoma among older Americans and to help combat this unfortunate trend.
Financial Disclosure: The authors have no significant financial interest or other relationship with the manufacturers of any products or providers of any service mentioned in this article.
References:
1. Jemal A, Tiwari RC, Murray T, et al: Cancer statistics, 2004. CA Cancer J Clin 54:8-29 2004.
2. Rigel DS: Melanoma update-2001. The Skin Cancer Foundation Journal 19:13-14, 2001.
3. Parker SL, Tong T, Boldern S, et al: Cancer statistics, 1996. CA Cancer J Clin 46:5-27, 1996.
4. Rigel DS, Carucci JA: Malignant melanoma: Prevention, early detection, and treatment in the 21st century. CA Cancer J Clin 50:215-236, 2000.
5. Koh HK: Cutaneous melanoma. N Engl J Med 325:171-182, 1992.
6. Kopf AW, Rigel DS, Freidman RJ: The rising incidence and mortality rate of malignant melanoma. J Dermatol Surg Oncol 8:760- 761, 1982.
7. Jemal A, Devesa SS, Hartge P, et al: Recent trends in cutaneous melanoma incidence among whites in the United States. J Nat Cancer Inst 93:678-683, 2001.
8. Geller AC, Miller DR, Annas GD,et al: Melanoma incidence and mortality among US whites, 1969-1999. JAMA 288:1719-1720, 2002.
9. Howe HL, Wingo PA, Thun MJ, et al: Annual Report to the Nation on the Status of Cancer (1973 through 1998), featuring cancers with recent increasing trends. J Natl Cancer Inst 93:824-42, 2001.
10. Demierre MF, Chung C, Miller DR, et al: Early detection of thick melanomas in the United States. "Beware" of the nodular subtype. J Natl Cancer Inst 2003; submitted.
11. Rhodes AR, Weinstock MA, Fitzpatrick TB, et al: Risk factors for cutaneous melanoma- A practical method of recognizing predisposed individuals. JAMA 258:3146-3154, 1987.
12. Sagebiel RW: Melanocytic nevi in histologic association with primary cutaneous melanoma of superficial spreading and nodular types: Effect of tumor thickness. J Invest Dermatol 100:3225-3255, 1993.
13. Stolz W, Schmoeckel C, Landthaler M, et al: Association of early malignant melanoma with nevocytic nevi. Cancer 63:550-555, 1989.
14. Williams ML, Sagebiel RW: Melanoma risk factors and atypical moles. West J Med 160:343-350, 1994.
15. Loggie B, Salve GR, Bean J, et al: Invasive cutaneous melanoma in elderly patients. Arch Dermatol 127:1188-1193, 1991.
16. Tsao H, Bevona C, Goggins W, et al: The transformation rate of moles (melanocytic nevi) into cutaneous melanoma: A populationbased estimate. Arch Dermatol 139:232-238, 2003.
17. Levine J, Kopf AW, Rigel DS, et al: Correlation of thicknesses of superficial spreading malignant melanomas and age of patients. J Dermatol Surg Oncol 7:311-316, 1981.
18. Friedman RJ, Rigel DS, Kopf AW: Early detection of malignant melanoma: The role of physician examination and self-examination of the skin. Cancer J Clin 35:130-151, 1985.
19. Sober AJ, Fitzpatrick TB, Mihm MC Jr, et al: Early recognition of cutaneous melanoma. JAMA 242:2795-2799, 1979.
20. Clark WH, Elder DE, Guerry D IV, et al: Model predicting survival in stage I melanoma based on tumor progression. J Natl Cancer Inst 81:1893-1904, 1989.
21. Buzaid AC, Moss MI, Balch CM, et al: Critical analysis of the current American Joint Committee on Cancer staging system for cutaneous melanoma and proposal of a new staging system. J Clin Oncol 15:1039-1051, 1997.
22. US Dept of Health and Human Services, Public Health Service: NIH consensus development conference on diagnosis and treatment of early melanoma. JAMA 268:1314-1319, 1992.
23. Langley RG, Fitzpatrick TB, Sober AJ: Clinical characteristics, in Balch CM, Houghton AN, Sober AJ, et al (eds): Cutaneous Melanoma, 3rd ed, pp 81-101. St Louis, Quality Medical Publishing, 1998.
24. MacKie RM, Young D: Human malignant melanoma. Int J Dermatol 23:433-443, 1984.
25. Balch CM, Soong SJ, Shaw HM, et al: An analysis of prognostic factors in 8,500 patients with cutaneous melanoma, in Balch CM, Houghton AN, Milton GW (eds): Cutaneous Melanoma, 2nd ed, pp 165-187. Philidelphia, JB Lippincott, 1992.
26. Bergenmar M, Ringborg U, Mansson Brahme E: Nodular histogenetic type-the most significant factor for thick melanoma: Implications for prevention. Melanoma Res 8:403-411, 1998.
27. Chamberlain AJ, Fritschi L, Giles GG, et al: Nodular type and older age are the most significant associations of thick melanoma in Victoria, Australia. Arch Dermatol 138:609- 614, 2002.
28. Swetter SM, Jung S, Harvell JD, et al: Increased proportion of lentigo maligna and lentigo maligna melanoma subtypes in the Veterans Affairs Palo Alto Health Care System and Stanford University Medical Center. J Invest Dermatol 119:245, 2002.
29. Weinstock MA, Sober AJ: The risk of progression of lentigo maligna to lentigo maligna melanoma. Br J Dermatol 116:303- 310, 1987.
30. Reintgen DS, McCarty KM Jr, Cox E, et al: Malignant melanoma in black American and white American populations. A comparative review. JAMA 248:1856-1859, 1982.
31. Paladugu RR, Winberg CD, Yonemoto RH: Acral lentiginous melanoma. A clinicopathologic study of 36 patients. Cancer 52:161- 168, 1983.
32. Jain S, Allen PW: Desmoplastic malignant melanoma and its variants: A study of 45 cases. Am J Surg Pathol 13:358-373, 1989. 33. Conley J, Lattes R, Orr W: Desmoplastic malignant melanoma (a rare variant of spindle cell melanoma). Cancer 28:914-936, 1971.
34. Austin PF, Cruse W, Lyman G, et al: Age as a prognostic factor in the malignant melanoma population. Ann Surg Oncol 1:487-494, 1994.
35. Levine J, Kopf AW, Rigel DS, et al: Correlation of thickness of superficial spreading malignant melanomas and ages of patients. J Dermatol Surg Oncol 7:311-316, 1981.
36. Balch CM, Soong SJ, Murad TM et al: A multifactorial analysis of melanoma: II. Progostic factors in patients with stage I (localized) melanoma. Surgery 86:343-351, 1979.
37. Day CL Jr, Lew RA, Mihm MC, et al: A multivariate analysis of prognostic factors for melanoma patients with lesions ≥ 3.65 mm in thckness: The importance of revealing alternative Cox models. Ann Surg 195:44-49, 1982.
38. Day CL Jr, Mihm MC, Sober AJ, et al: Prognostic factors for melanoma patients with lesions 0.76-1.69 mm in thickness. An appraisal of "thin" level IV lesions. Ann Surg 195:30- 34, 1982.
39. Day CL Jr, Mihm MC, Lew RA: Prognostic factors for patients with clinical stage I melanoma of intermediate thickness (1.51-3.99 mm): A conceptual model for tumor growth and metastases. Ann Surg 195:35-43, 1982.
40. Balch CM, Soong SJ, Gershenwald JE, et al: Prognostic factors analysis of 17,600 melanoma patients: Validation of the American Joint Committee on Cancer Melanoma Staging System. J Clin Oncol 19, 3622-3634, 2001.
41. Walford RL: Immunology and aging. Am J Clin Pathol 74:247-253, 1980.
42. Morris BT, Sober AJ: Cutaneous malignant melanoma in the older patient. Dermatologic Clin 4:473-480, 1986.
43. Christos PJ, Oliveria SA, Berwick M, et al: Signs and symptoms of melanoma in older populations. J Clin Epidem 53:1044-1053, 2000.
44. Cassileth BR, Lusk EJ, Guerry D IV, et al: "Catalyst" symptoms in malignant melanoma. J Gen Intern Med 2:1-4, 1987.
45. Hanrahan PF, Hersey P, D’Este CA: Factors involved in presentation of older people with thick melanoma. Med J Aust 169:410-414, 1998.
46. Hersey P, Sillar RW, Howe CG, et al: Factors related to the presentation of patients with thick primary melanomas. Med J Aust 154:583-587, 1991.
47. Hanrahan P, Hersey P, Watson AB: The effect of an educational brochure on knowledge and early detection of melanoma. Aust J Public Health 19:270-274, 1995.
48. Islam M, Frye RF, Richards TJ, et al: Differential effect of IFNalpha-2b on the cytochrome P450 enzyme system: A potential basis of IFN toxicity and its modulation by other drugs. Clin Cancer Res 8:2480-2487, 2002.
49. Kirkwood JM, Bender C, Agarwala S, et al: Mechanisms and management of toxicities associated with high-dose interferon alfa- 2b therapy. J Clin Oncol 20:3703-3718, 2002.
50. American Academy of Dermatology: 2003 Melanoma/Skin Cancer Screening Program, Schaumburg, Ill, 2003.
51. United States Preventive Services Task Force: Screening for skin cancer. Am J Prev Med 20(3S):44-46, 2001.
52. Institute of Medicine: Extending Medicare coverage for prevention and other services. Washington, DC, National Academies Press, 2000.
53. McDonald CJ: American Cancer Society perspective on the American College of Preventive Medicine’s policy statements on skin cancer prevention and screening. CA Cancer J Clin 48:232-235, 1998.
54. de Rooij, Rampen FH, Schouten LF, et al: Skin cancer screening focusing on melanoma yields more selective attendance. Arch Dermatol 131:422-425, 1995
55. Katris P, Donovan RJ, Gray BN: The use of targeted and non-targeted advertising to enrich skin cancer screening samples. Br J Dermatol 135:268-274, 1996
56. Swetter SM, Waddell BL, Vazquez MD, et al: Increased effectiveness of targeted skin cancer screening in the Veterans Affairs population of northern California. Prev Med 35:164- 171, 2003.
57. Geller AC, Sober AJ, Zhang Z, et al: Strategies for improving melanoma education and screening for men age 50+ years: Findings from the American Academy of Dermatology National Skin Cancer Screening Program. Cancer 95:1554-1561, 2002.
58. Santmyire BR, Feldman SR, Fleischer AB Jr: Lifestyle high-risk behaviors and demographics may predict the level of participation in sun-protection behaviors and skin cancer primary prevention in the United States: Results of the 1998 National Health Interview Survey. Cancer 92:1315-1324, 2001.