Modified Hyper-CVAD Reduces Induction Mortality in Older ALL Patients
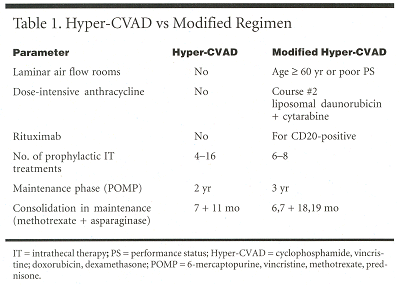
HOUSTON-A modified "hyper-CVAD" (cyclophosphamide [Cytoxan, Neosar], vincristine, doxorubicin [Adriamycin], dexamethasone) regimen, which included the addition of rituximab (Rituxan) for CD20-positive patients and the use of laminar airflow rooms for patients who were elderly or had poor performance status, reduced induction mortality in older patients with acute lymphoblastic leukemia (ALL). Overall mortality was not changed, because a number of patients died of other causes while in complete remission (CR).
HOUSTONA modified "hyper-CVAD" (cyclophosphamide [Cytoxan, Neosar], vincristine, doxorubicin [Adriamycin], dexamethasone) regimen, which included the addition of rituximab (Rituxan) for CD20-positive patients and the use of laminar airflow rooms for patients who were elderly or had poor performance status, reduced induction mortality in older patients with acute lymphoblastic leukemia (ALL). Overall mortality was not changed, because a number of patients died of other causes while in complete remission (CR).
Deborah A. Thomas, MD, presented a poster at the 43rd Annual Meeting of the American Society of Hematology describing outcomes in 71 patients with newly diagnosed or primary refractory ALL treated with the modified regimen (see Table 1). She is assistant professor in the Department of Leukemia at The University of Texas M. D. Anderson Cancer Center in Houston.
Induction-Related Toxicity Was Significant
The reported CR rate with hyper-CVAD in an earlier study was 90%, and the 3-year disease-free survival rate was 38%, but at the cost of significant induction-related toxicity. The M. D. Anderson investigators developed a modified hyper-CVAD regimen in the hope of reducing induction mortality, which was 17% in patients aged 60 or older vs 3% in younger patients. The researchers also hoped to lengthen disease-free survival by using early anthracycline intensification and by using rituximab to counter the worse survival associated with CD20 expression. They also hoped to reduce the previously reported central nervous system (CNS) relapse rate of 6% and 1% in low- and high-risk patients, respectively, and to reduce the number of late relapses.
Modified Regimen
From May 2000 to July 2001, 71 newly diagnosed or primary refractory patients were treated with the modified regimen. These had a median age of 44 years (range: 18-83), with 27% over 60. Forty-two percent were CD20-positive, and 8% had the t(9;22) marker.
The overall response rate was 98%. There was only one death related to induction therapy. It was due to extensive tumor infiltration of bowel with perforation following induction therapy. Dr. Thomas said that this showed that adding rituximab to the regimen did not appear to increase myelosuppressive toxicity. With a median follow-up of 5 months, there were relapses in seven patients (10%), including two isolated CNS relapses at 25 and 28 weeks despite aggressive intrathecal therapy treatment. Five patients had systemic relapses, all of whom were younger patients with high-risk features, according to Dr. Thomas.
The 1-year disease-free survival rate was 71% overall (compared with 49% for the original hyper-CVAD regimen, P = .03). One-year DFS was 100% for patients with CD20-positive ALL vs 61% for CD20-negative disease.
Twenty-nine percent of patients over 60 years old died in CR vs 2% of younger patients (P = .001). Deaths in older patients were related to pneumonia in four patients, sudden death in one patient, and failure to thrive in one patient.
Median Follow-up Is Encouraging
In patients with CD20-positive ALL at a median follow-up of 6 months, treatment failure occurred in 1 of 24 (5%) patients on modified hyper-CVAD including rituximab, compared to previously reported data showing failure in 18/36 (50%) patients on conventional hyper-CVAD without rituximab.
"The reduction in early mortality is encouraging. However, strategies such as additional dose modifications are needed to reduce infectious complications in elderly patients postremission. But monitoring for CNS relapse indicates that failures were in patients with CNS disease at presentation and occur with concomitant systemic relapse. Rituximab plus hyper-CVAD is a feasible regimen that may improve long-term outcome in CD20-positive ALL," Dr. Thomas concluded.