PERSPECTIVE
John L. Marshall, MD, on the role of molecular profiling in transforming global cancer care at
ABSTRACT: Colorectal cancer (CRC) is a commonly diagnosed malignancy. Although chemotherapy remains the backbone of treatment, the landscape of treating metastatic CRC (mCRC) is changing with the understanding of its heterogeneity and molecular blueprint. Colon cancer sidedness has proven to hold prognostic implications, with right-sided tumors having higher incidence of BRAF and KRAS mutations and being microsatellite instability–high (MSI-H); overall, they have a worse prognosis compared with left sided-tumors. Results of molecular research have demonstrated the need to profile each mCRC patient for RAS and BRAF mutations, MSI-H status, HER2 amplifications, and NTRK fusions. Ongoing clinical trials using targeted agents aim to further improve survival outcomes. We emphasize the epidemiology, knowledge of primary tumor location, and mutational landscape of mCRC, as well as novel treatment options for patients harboring unique subtypes of these characteristics.
Colorectal cancer (CRC) is a commonly diagnosed malignancy and the third leading cause of cancer death in the United States, with 147,950 estimated new cases and 53,200 estimated deaths in 2020.1 Despite improvements in CRC screening methodologies, approximately 20% of patients are diagnosed with metastatic CRC (mCRC), which carries a 14.2% 5-year survival rate.2 Although patients with stage IV CRC can occasionally undergo curative metastasectomies, treatment of patients with mCRC is for the most part palliative in nature and aims to prolong and improve the quality of life. A better understanding of the heterogeneity of mCRC, including primary tumor location, microsatellite instability (MSI) status, and other clinically actionable tumor mutations, is reshaping the therapeutic landscape. Here we review the current molecular profiling practice for mCRC along with its prognostic implications and potential therapeutic targets.
Colon tumor sidedness has been a topic of great interest. The activated pathways in mCRC differ between the left and right sides.3 The difference in sidedness may stem from embryonic origin: the right side of the colon arises from the midgut, whereas the left side arises from the hindgut, affecting blood supply.4 Differences in gut microbiome, related to increased bile acid exposure on the right side and predominant bacteria on the 2 sides of the colon—including Prevotella, Pyramido-bacterium, Selenomonas, and Peptostreptococcus (right); and Fusobacterium, Escherichia–Shigella, and Leptotrichia (left)—may all play a role in the phenotype of CRC sidedness.4 The right side of the colon includes the cecum through to the proximal two-thirds of the transverse colon, and the left side of the colon includes the distal third of the transverse colon through to the sigmoid colon.4 Most studies define right-sided tumors as those that arise from the cecum through but not including the splenic flexure, and left-sided tumors are those found in the splenic flexure through the sigmoid colon.5 Rectal cancers are frequently included with left-sided colon cancers for the purpose of sidedness analyses.
The results of many studies have shown that patients with left-sided colon tumors tend to have superior overall survival (OS) compared with patients with right-sided tumors.6-10 The CALGB/SWOG 80405 trial results revealed that the addition of cetuximab (an anti-EGFR monoclonal antibody) vs bevacizumab (an anti-VEGF monoclonal antibody) to standard chemotherapy in the first-line treatment of patients with KRAS exon 2 wild-type (WT) mCRC did not improve OS, necessitating further study by dividing these patients into subsets to discover how best to direct standard therapeutic recommendations.11 A follow-up study utilizing the same patient population (N = 782) confirmed primary tumor location as an independent prognostic factor for OS, with right-sided tumors being associated with inferior survival (HR, 1.392; 95% CI, 1.032-1.878; P = .031).11 Right-sided compared with left-sided colorectal tumors were found to have a higher BRAF mutation frequency and were more likely to be MSI-high (MSI-H).12,13
The NCIC CO.17 trial (N = 399) demonstrated that right-sided colon tumors (N = 150) were more poorly differentiated and had a higher rate of KRAS mutations than left-sided tumors.5 For patients with KRAS WT tumors only, those with left-sided tumors had an increased progression-free survival (PFS) benefit from cetuximab compared with best supportive care (median PFS, 5.4 vs 1.8 months; HR, 0.28; P <.0001), whereas those with right-sided tumors received no such benefit (median 1.9 vs 1.9 months; HR, 0.73; P = .26).5 Investigators from other trials also identified a response to chemotherapy plus anti-EGFR therapy in patients with left-sided BRAF/RAS WT tumors, while those with right-sided tumors had adverse outcomes to anti-EGFR therapy but benefited from chemotherapy plus VEGF inhibition.5,8,11,14-16
TABLE. Alterations, Frequency, Sidedness, and Targeted Therapies
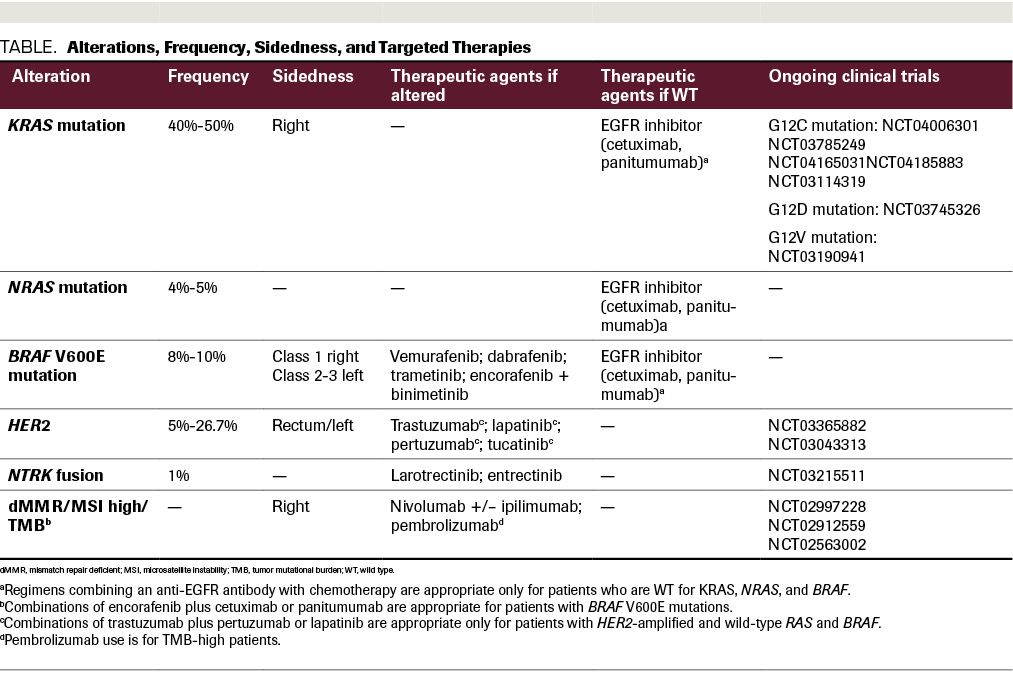
Mutations in any 1 of 3 RAS oncogenes—KRAS, NRAS, and HRAS—have been found to lead to autonomous growth and differentiation of cells driving cancer progression. A recent study of CRC patients (N = 13,336) identified KRAS mutations in 51.9% of specimens, with NRAS and HRAS mutations occurring at lower rates.17 These study results revealed that KRAS-mutated colorectal tumors were less likely to be mismatch repair deficient (dMMR).17
The presence of KRAS or NRAS mutations correlates with worse outcomes and resistance to anti-EGFR therapy, with or without chemotherapy.17-22 Investigators in the phase 3 PRIME trial (N = 1183) randomized patients with treatment-naïve mCRC to receive panitumumab plus FOLFOX (5-FU, leucovorin, and oxaliplatin) or FOLFOX alone.23 This study demonstrated that in patients with KRAS/NRAS WT disease, the addition of panitumumab to FOLFOX resulted in superior median PFS (10.1 vs 7.9 months; HR, 0.72; P = .004) and median OS (26.0 vs 20.2 months; HR, 0.78; P = .04). BRAF mutations were a negative prognostic and predictive factor, as patients with BRAF mutations did not benefit from panitumumab.23
RAS mutations, specifically KRAS and NRAS mutations, are used as predictive biomarkers for therapeutic resistance to upstream EGFR inhibitors. However, the successful development of targeted therapies to KRAS and other downstream signaling proteins means that KRAS mutation testing may play another role. Multiple phase 1 trials are testing small molecule agents that target the KRAS glycine-to-cysteine (G12C)–mutated protein (NCT04006301, NCT03785249, NCT04165031, NCT04185883). In addition to direct mutational targeting, agents targeting SHP2—which, encoded by PTPN11, is needed to activate RAS-MAPK—may become a viable therapeutic option for KRAS G12C–mutated CRC (NCT03114319). Additional targeted KRAS therapies include lymphocytes genetically engineered to express murine T-cell receptors that recognize G12V and G12D mutations (NCT03745326, NCT03190941).
Similar to the effects of KRAS mutation, patients with BRAF-mutated mCRC do not respond to anti-EGFR monotherapy (panitumumab or cetuximab) and have shorter PFS and OS compared with patients with BRAF WT tumors.19,24 Approximately 8% to 10% of mCRCs contain a mutation in BRAF, a gene that encodes a signal transduction protein that is involved in the MAPK pathway.25,26
Due to its link to poor patient prognosis, the BRAF V600E mutation is a worthy target in CRC. However, BRAF inhibition alone has limited efficacy in the treatment of patients with mCRC. Vemurafenib is a small molecule tyrosine kinase inhibitor (TKI) that specifically targets the ATP-binding domain of BRAF V600E, hence activating the MAPK pathway in advanced malignancies with BRAF V600E mutations.26 In mCRC, vemurafenib monotherapy is rarely effective due to incomplete inhibition of MAPK signaling and a reflexive activation of EGFR.26 Kopetz and colleagues studied the efficacy of irinotecan plus cetuximab with or without vemurafenib in 106 patients with refractory BRAF-mutated mCRC.27 PFS was improved with the addition of vemurafenib (median 4.4 vs 2.0 months; P <.001), and there was a trend toward increased response rate (16% vs 4%) and disease control rate (67% vs 22%). Hence, vemurafenib plus irinotecan and cetuximab provided clinical benefit and became a new treatment option for this subset of patients.27
In addition to combined inhibition of EGFR and BRAF, the inhibition of the MAPK pathway has been explored through the combined inhibition of MEK, EGFR, and BRAF. The phase 3 BEACON study randomized patients with previously treated BRAF V600E–mutated mCRC 1:1:1 to triplet therapy of encorafenib (a BRAF inhibitor), cetuximab, and binimetinib (a MEK inhibitor); doublet therapy with encorafenib and cetuximab; or the control arm of chemotherapy (FOLFIRI or irinotecan) with cetuximab (N = 665).28 Patients treated with the triplet and doublet had significant longer median OS when compared with the chemotherapy arm: 9.3 and 9.3 vs 5.9 months, respectively. Objective response rate (ORR) was also higher with the triplet (27%) and doublet (20%) compared with the chemotherapy arm (2%; P <.0001).28 As there was not a statistical numerical difference in terms of OS between the triplet and doublet, encorafenib and cetuximab (without binimetinib) was approved by the FDA.29 The ongoing phase 3 ANCHOR trial is evaluating the triplet in the first-line setting.
John L. Marshall, MD, on the role of molecular profiling in transforming global cancer care at
The PD-1 pathway is a negative feedback system that regulates cytotoxic Th1 immune response to prevent damage to host cells.30 Tumor cells upregulate the PD-1 receptor or PD-1 ligand (PD-L1 or PD-L2) to evade destruction by the host’s immune system. The PD-1 pathway has been exploited in cancer immunotherapy. By blocking the PD-1 pathway with antibodies to PD-1 or PD-L1/PD-L2, the adaptive immune response is activated against tumor cells, resulting in an anticancer response. This has been observed in patients with a number of advanced cancers, including melanoma, non–small cell lung cancer, renal cell carcinoma, and others.30 Further exploration of the PD-1 pathway has demonstrated the connection between response to PD-1 immune checkpoint inhibitors and dMMR. CRC with dMMR or MSI-H have a poor response to chemotherapy regimens, resulting in a worse prognosis for patients with metastatic disease.31
Tumors with dMMR contain large numbers of mutation-associated neoantigens; the immune system is more likely to recognize these antigens as foreign and launch an immune response. The clinical benefit of PD-1 blockade with pembrolizumab (an anti–PD-1 antibody) was first documented in a phase 2 study (N = 41), which enrolled patients with metastatic carcinoma with and without dMMR, and results revealed that only those with dMMR benefit from PD-1 blockade. Eleven patients with dMMR had CRC: 4 patients achieved a partial response and 5 had stable disease.30 In a larger study of this population, the ORR of patients with dMMR CRC (N = 40) was 52%, with a 2-year PFS of 59%.32 Of the posttreatment biopsies obtained and analyzed (N = 20), 12 showed inflammation, fibrosis, and mucin, but no evidence of tumor cells. This demonstrated an immunological anticancer response to PD-1 inhibitor therapy.32 The phase 2 KEYNOTE-164 trial evaluated pembrolizumab in larger cohort of 124 patients with metastatic MSI-H/dMMR mCRC and demonstrated an ORR of 33%.33 Additionally, analysis of tumor mutational burden in the phase 2 KEYNOTE-158 trial led to the FDA approval of pembrolizumab across solid tumors harboring a tumor mutation burden (TMB) of 10 or more mutations/megabase after progression on prior therapy and showed a response rate of 29%.34,35
The anti–PD-1 antibody nivolumab was also found to be an effective therapy for patients with MSI-H/dMMR mCRC. Investigators of the CheckMate 142 trial (N = 74) reported ORRs, by RECIST 1.1, of 31.1%. Disease control rate at >12 weeks was found to be 68.9%.31 Of note, tumor expression of PD-L1 was not found to be predictive of response because an objective response and disease control were also found in subjects with and without PD-L1 expression.31 Additionally, nivolumab and other checkpoint inhibitors may exhibit greater activity than conventional chemotherapy or targeted therapy in patients with MSI-H, BRAF-mutant tumors, as shown by unprecedented response rates of 25% in patients with BRAF-mutant tumors.31 Evidence from nivolumab and pembrolizumab studies indicates that these agents have efficacy in mCRC with MSI-H activity/dMMR.
To further enhance the possible therapeutic benefits of immunotherapy, the CheckMate 142 study also explored combination therapy with nivolumab and ipilimumab. Ipilimumab is an antibody targeting the CTLA-4 receptor; combination therapy with an anti–PD-1 antibody was shown to be more active than nivolumab monotherapy. Combination therapy appeared to provide a higher response rate (55%) relative to that of the monotherapy group (31%).36 At 12 months, nivolumab plus ipilimumab had longer survival (PFS, 71%; OS, 85%) than nivolumab alone (PFS, 50%; OS, 73%).36
To investigate the therapeutic benefit of PD-1 immunotherapy in the first-line setting for patients with dMMR or MSI-H mCRC, the KEYNOTE-177 trial is currently ongoing (NCT02563002). The PFS data were presented at the American Society for Clinical Oncology 2020 Virtual Scientific Program, and there was a clear improvement with pembrolizumab with a doubling of median PFS over investigator’s choice of chemotherapy (16.5 vs 8.2 months; HR, 0.60; 95% CI, 0.45-0.80; P = .0002) and only one-third of the grade 3-5 adverse event rate (22% vs 66%).37 These findings have changed the standard of care to immunotherapy in the first-line setting for patients with dMMR or MSI-H mCRC and recently resulted in FDA approval.35 The COMMIT study is another randomized, open-label phase III study that compares mFOLFOX6/bevacizumab vs atezolizumab monotherapy vs mFOLFOX6/bevacizumab plus atezolizumab as first-line interventions for patients with dMMR/MSI-H mCRC (NCT02997228).
Gene amplification of HER2, also referred to as ERBB2, is well described in breast and gastric cancers. Recently, HER2 amplification emerged as a therapeutic target in mCRC. HER2 overexpression is defined by immunohistochemistry (IHC) and amplification by fluorescence in situ hybridization (FISH). By IHC, colorectal tumor samples that show intense staining (3+) in greater than 50% of cells are designated HER2-positive. By FISH, an ERBB2:CEN17 ratio of 2.0 or less in 50% or more of cells (when counting ERBB2 and CEN17 signals from 100 nuclei per case) indicates HER2 amplification.38 Using this analytical methodology, the incidence of HER2/neu amplification in mCRCs overall is low (approximately 5%). However, when analyzing mCRCs with a known rectal primary, the incidence can range from 12.4% to 26.7%.38-40
The open-label phase 2 HERACLES trial enrolled 27 patients with KRAS exon 2 WT, HER2-positive mCRC who were treated with the HER2 monoclonal antibody trastuzumab and the dual HER2/EGFR TKI lapatinib.41 Results revealed an ORR of 30%, median PFS of 21 weeks (95% CI, 16-32), median OS of 46 weeks (95% CI, 33-68), and manageable toxicities.41 In a follow-up analysis, the plasma copy number of ERBB2 amplifications was assessed in 48 plasma samples from 29 of the HERACLES trial’s HER2-positive patients.42 The MyPathway phase 2 basket trial enrolled a subset of patients with treatment-refractory HER2-positive mCRC (N = 57) and treated them with dual HER2-targeted therapy, trastuzumab and pertuzumab.43 The treatment regimen was generally well tolerated and the response rate was 32%.43 Ongoing clinical trials are further investigating HER2-targeted therapies vs standard chemotherapy (NCT03365882, NCT03043313). The novel antibody–drug conjugate trastuzumab deruxtecan showed a promising 45.3% response rate in a heavily pretreated patient population with HER2-positive mCRC.44
NTRK gene fusions, which encode TRKs, are rare but highly actionable. NTRK fusions lead to oncogenic overexpression of chimeric oncoproteins, which independently activate downstream signaling.45 One multiphase trial explored the ORRs of TRK fusion–positive cancer patients (N = 55) to larotrectinib, a highly selective TRK inhibitor.46 Seven percent (N = 4) of the patients treated had CRC, and their ORR was 75% (95% CI, 61%-85%), with durable responses seen at 1 year.46 The results from two phase 1 trials, ALKA-372-001 and STARTRK-1, which tested the safety and efficacy of entrectinib, a multitargeted pan-TRK, ROS, and ALK inhibitor, in the treatment of TKI treatment–naïve patients with advanced or metastatic solid tumors, were reported together; they revealed that entrectinib was well tolerated and led to durable clinical responses lasting as long as 2 years.47 Of the 119 patients treated, 15% had gastrointestinal primary tumors (N = 18). In the phase 2 portion of these studies, 1 patient with mCRC and an NTRK fusion had disease response lasting 2.6 months, which demonstrated a potential benefit to targeting TRK in mCRC. Acquired TRK resistance mechanisms known as solvent front mutations can develop. Trials exploring next-generation TRK inhibitors such as LOXO-195/selitrectinib to overcome these resistance mechanisms are ongoing.48
mCRC remains a leading cause of cancer death in the United States, but research is beginning to show that alteration of the course of this disease may be possible with innovative interventions. Chemotherapy has provided reliable initial treatment, and molecular pro ling is creating new therapeutic options that give patients hope of increased survival. The research community has demonstrated the need to pro le patients with mCRC for RAS and BRAF mutations, MSI-H status, HER2 amplifications, and NTRK fusions. Knowledge of primary tumor location along with specific targetable tumor mutations is beginning to change the outlook for patients with mCRC.
Weinberg is an assistant professor of medicine, Ruesch Center for the Cure of Gastrointestinal Cancers, Lombardi Comprehensive Cancer Center, Georgetown University Medical Center

Armstrong is a third year medical hematology/oncology fellow at Georgetown Lombardi Comprehensive Cancer Center

Malley is a third year medical student at the Georgetown University School of Medicine

