More Evidence to Support Capecitabine/Oxaliplatin for Advanced Colorectal Cancer
DETROIT-A phase II trial of capecitabine (Xeloda) plus oxaliplatin (Eloxatin, investigational in the United States) supports European data suggesting that the combination is active in advanced colorectal cancer, and with manageable toxicity.
DETROITA phase II trial of capecitabine (Xeloda) plus oxaliplatin (Eloxatin, investigational in the United States) supports European data suggesting that the combination is active in advanced colorectal cancer, and with manageable toxicity.
Anthony Shields, MD, PhD, professor of medicine and oncology, Barbara Ann Karmanos Cancer Institute, Detroit, reported the results at the 38th Annual Meeting of the American Society of Clinical Oncology (abstract 568).
The investigators planned a two-stage trial with an accrual goal of 35 patients if at least 3 of the first 17 patients responded. After treating 13 patients, however, they opted to make a toxicity-related dose reduction in the capecitabine. An additional 35 patients were entered into the study and treated at the lower dose, for a total of 48 treated patients overall.
The median age of the patients was 60. Twenty-eight had colon cancer, and 20 rectal cancer; 18 had received adjuvant chemotherapy. Most (45) were ECOG performance status 0-1.
The initial 13 patients received capecitabine 1,000 mg/m² bid on days 1 to 14 of a 21-day cycle and 130 mg/m² of oxaliplatin by infusion on day 1. "We found that we couldn’t deliver the 2,000 mg/m² dose of capecitabine because of toxicity," he said. "So we cut the dose down to 1,500 mg/m² per day [750 mg/m² bid]."
Five (38.4%) of the 13 patients given the higher starting dose responded. Within the first two cycles, however, six patients were hospitalized for toxicityfive with diarrhea and dehydration. Six of these 13 patients have now experienced disease progression.
Of the patients who received the lower dose, 14 of 35 (40%) had a par-tial response. The median estimated progression-free survival was 6.9 months (range, 4.4 to 8.9). Sixteen patients have progressed, and one has died.
The toxicity has been somewhat higher than in the larger European trial, Dr. Shields said. Among patients on the lower dose, 10 have been hospitalized, 3 with diarrhea/dehydration, 3 with abdominal pain, and 1 each with febrile neutropenia, small bowel obstruction, pulmonary embolism, and cardiac arrest and seizure. One of the patients with diarrhea developed sepsis and died.
The predominant toxicity was diarrhea (see table below). Overall, six patients developed grade 3-4 diarrhea at the lower dose, and two developed grade 3-4 neutropenia.
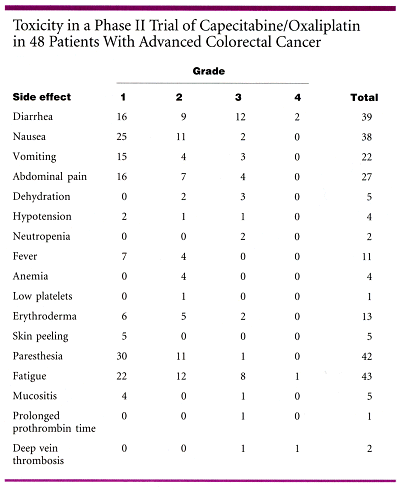
Dr. Shields said that the convenience and activity of this regimen warrant formal comparisons with the infusional fluorouracil (5-FU)/oxaliplatin regimen (FOLFOX). Compared with infusional treatment regimens, including the 5-FU/irinotecan combination (FOLFIRI), the capecitabine/oxaliplatin regimen is simple to give, reduces patient time in the hospital, and essentially allows the patient to see the doctor once every 3 weeks.
Dr. Shields added that he suspects FOLFOX, FOLFIRI, and the capecitabine/oxaliplatin regimens will all turn out to be fairly close in terms of efficacy. "The deciding issues will be which regimen is best for the individual patient and which is the easiest for a patient to take," he said.