A Multidisciplinary Approach to Perianal and Intra-Abdominal Infections in the Neutropenic Cancer Patient
The focus of this review will be the multidisciplinary approach to management of anorectal infection, neutropenic enterocolitis, appendicitis, and cholecystitis in the neutropenic cancer patient.
Figure 1: CT Images From Patients With Neutropenia-Related Perianal Infections
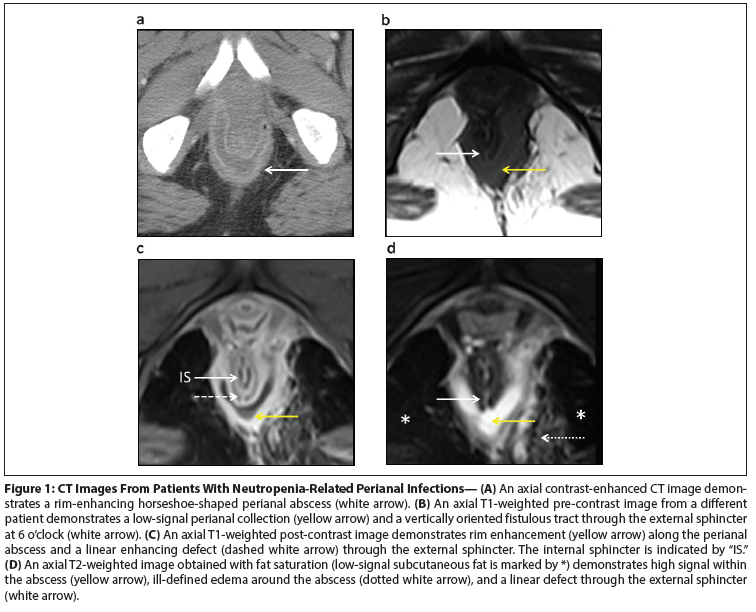
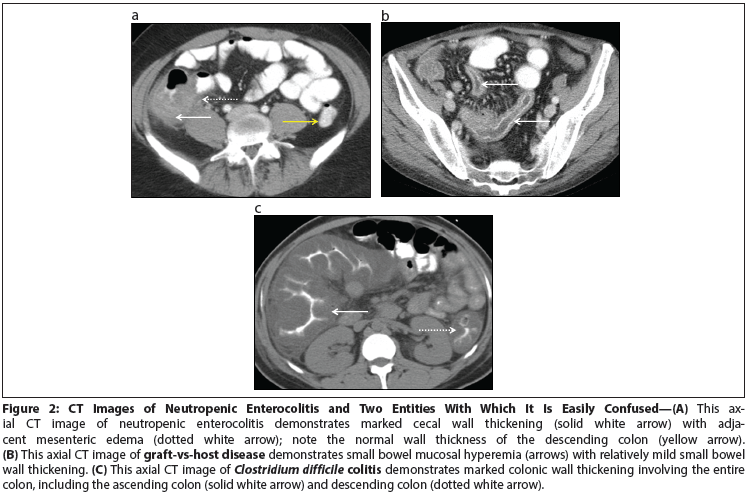
Figure 2: CT Images of Neutropenic Enterocolitis and Two Entities With Which It Is Easily Confused
The chemotherapeutic treatment of both hematologic and solid organ malignancies has increased in recent decades, resulting in increased neutropenia-related perianal and intra-abdominal infections. Nearly 30% of neutropenia-related infections arise in the gastrointestinal tract. The management of these patients is often not straightforward, and the indications for and timing of surgical intervention continue to be unclear. The management strategy must take into account such factors as recent chemotherapy toxicity, stage and prognosis of the malignancy, performance status, comorbidities, degree of neutropenia, immunosuppression, thrombocytopenia, and corticosteroid use. The degree and duration of neutropenia is a key determinant of infection resolution. The focus of this review will be the multidisciplinary approach to management of anorectal infection, neutropenic enterocolitis, appendicitis, and cholecystitis in the neutropenic cancer patient.
Anorectal Infection
Background
Anorectal infection in the neutropenic cancer patient is a significant and potentially fatal complication in patients receiving systemic chemotherapy. The diagnosis is often made clinically on the basis of signs and symptoms (perianal pain, erythema, and tenderness); after surgical drainage of an abscess; or from cross-sectional imaging demonstrating perianal inflammation, fluid collection, or fistula formation. The management of these patients is not straightforward, and the literature continues to be unclear about the indications for and timing of surgical intervention. Early reports of neutropenic patients with perianal infection showed mortality rates as high as 50%.[1,2] The mortality rates have decreased in modern series, with most patients recovering with supportive care, antimicrobial therapy, and the selective use of surgical intervention. Although there has been an improvement in outcomes, determining the optimal medical management, as well as the indications for and appropriate timing of surgical intervention, remains a challenge for clinicians.
Incidence of and risk factors for neutropenia-related anorectal infection
The incidence of perianal infection in patients with hematologic malignancies is between 5% and 9%.[3] Although the incidence of such infections in patients with soft-tissue and solid organ malignancies is unknown, the incidence of perianal infection in cancer patients with neutropenia is higher than that in the general population, which has been estimated to be approximately 100,000 cases per year in the United States.[4]
One of the most significant risk factors for perianal infection is neutropenia.[2,3,5-8] Neutrophils are an essential component of the immune response, playing a key role in inflammation, localization, and pus formation.[6] Chemotherapy regimens resulting in myelosuppression are more common in the treatment of hematologic malignancy than in solid organ malignancy. Ablative regimens used in preparation for bone marrow transplant can also cause profound pancytopenia, placing patients at risk for neutropenia-associated infections. In patients with hematologic malignancies, male sex and age younger than 40 years are also risk factors for perianal infections.[3,6,9,10]
Pathogenesis of perianal infection in the neutropenic cancer patient
The pathogenesis of perianal infection in the neutropenic patient is similar to the pathogenesis in an immunocompetent patient. A combination of factors, including impaired host defense against invasion of microorganisms, profound neutropenia, and mucosal injury from cytotoxic drugs, can all contribute to increased rates of perianal infection.[11] Anorectal abscesses arise in crypt glands in the anal canal surrounding the dentate line (pectinate line).[12] The dentate line is the visible transition in the anal canal from the proximal columnar epithelium of the rectum to the squamous epithelium of the perianal skin. The surgical anal canal is approximately 3 cm in length from the anal verge (or internal sphincter) to the proximal levator ani (at the level of the puborectalis muscle). An average of six glands lined by stratified columnar epithelium with mucus-secreting or globlet cells open into crypts surrounding the anal canal. Infection occurs when an anal crypt is obstructed by inspissated debris and bacterial overgrowth develops. The inflammatory response typically begins with a neutrophil response, followed by formation of pus and an abscess collection. The abscess collection follows the path of least resistance and terminates where the anal glands penetrate the sphincter complex. The resulting abscess can potentially form in a number of perianal spaces: submucosal, high intermuscular, supralevator, ischiorectal, or perianal locations.[12] As the infection matures, it may resolve with an immune response or it may grow larger, causing pain, swelling, and drainage.
The infection may continue to grow into a defined collection in one of the potential spaces of the sphincter complex. The collection location will depend on the extent of penetration of the infected gland into surrounding tissues and the path of least resistance; possible locations include supralevator, submucosal/intermuscular, intersphincteric, ischiorectal, or perianal.[4] A drained abscess may heal permanently, may heal and recur at the same location, or may continue to drain intermittently or continuously.[4] A drainage tract may develop, with communication between the anal crypt gland and the skin of the buttock. The resulting formation-a tract between two epithelial structures-is referred to as a fistula-in-ano. There is no means of predicting whether an abscess will result in a fistula-in-ano. The vast majority of perianal abscesses are cryptoglandular in origin, and if the chronic component of a fistula-in-ano results, the internal opening is frequently located at the dentate line.
It is important to anticipate that approximately 30% to 40% of patients will develop a fistula-in-ano after drainage of a perianal abscess.[13] The location of the drained abscess will determine the chance of a fistula developing. Intersphincteric abscesses have been found to have the highest association with a post-drainage fistula.[13] The perianal abscess is the acute component of the infection, and the fistula-in-ano represents the chronic component. Fistulae-in-ano are commonly classified as intersphincteric, transphincteric, suprasphincteric, and extrasphincteric.[4]
Diagnosis of perianal infection in a neutropenic patient, including use of imaging
Typical signs of infection include fever (temperature > 38°C); perianal pain; and physical examination findings of perianal fullness, induration, erythema, fluctuance, and possibly purulent drainage. However, the clinician may not visualize obvious signs of infection that would otherwise be present in an immunocompetent patient. Neutropenic patients may have redness and induration but lack fluctuance because of their low neutrophil counts.[3,14] Although the lack of a fluctuant abscess has been associated with low neutrophil counts, some authors have reported drainage of purulence from perianal abscesses in neutropenic patients. In a cohort of 100 patients presenting with perianal infection and neutropenia, over 90% had perianal pain, 30% had swelling, and 25% had perianal drainage.[14] Most patients present with perianal pain and discomfort for 2 to 3 days prior to presentation.[14] Symptoms often begin as the neutrophil count nadirs, usually 7 to 14 days after cytotoxic chemotherapy.
If the diagnosis is in question in a patient with perianal pain and a paucity of clinical signs, then imaging is warranted. We prefer to image with contrast-enhanced MRI. Although large perianal abscesses will be visible on a CT scan, the superior soft-tissue resolution of MRI allows for better delineation of fistulous tracts and their relationship to the internal and external anal sphincters (Figure 1A–D).[15]
On pre-contrast T1-weighted MRI images, perianal abscesses typically demonstrate low signal (Figure 1B). T1-weighted post-contrast images are helpful for demonstrating rim enhancement around fluid collections and for demonstrating enhancing walls of fistulous tracts (Figure 1C). T2-weighted images obtained with fat saturation best demonstrate fluid collections and related edema, which will appear as areas of high signal against a background of low-signal fat (Figure 1D). Finally, MRI correctly classified the location of fistulous tracts in 90% of 104 patients and can aid in operative management.[16]
Management of perianal infection in the neutropenic cancer patient
The routine treatment of a perianal abscess in an immunocompetent patient is simple incision and drainage, and in the vast majority of patients, no antimicrobial therapy is needed. In cancer patients, the need for further cytotoxic chemotherapy, continued immunosuppression following a bone marrow transplant, neutropenia, thrombocytopenia, corticosteroid use, patient comorbidities, and ethical issues surrounding the stage and prognosis of the malignancy all influence decision making. The literature has many single-center experiences that advocate surgical or nonsurgical approaches. No level I evidence exists to advise clinicians on the best treatment in these complicated scenarios. Historic cohorts of neutropenic cancer patients presenting with perianal infections have reported mortality rates as high as 50%.[1,2] More recent series report improved mortality (0% to 16%), likely due to improvements in critical care, a multidisciplinary approach, targeted microbial coverage, selective use of surgical intervention, and the use of a colony-stimulating factor, which have all contributed to better outcomes.[17]
After diagnosing a perianal infection, it is important to use a multidisciplinary approach when determining the treatment strategy. This often involves collaboration between the medical oncologist, a general surgeon, and an infectious disease specialist. After other potential sources of infection are ruled out and cultures have been obtained (blood and urine), the patient should be started immediately on antimicrobial therapy. Coverage should include broad coverage of gram-positive, gram-negative, and anaerobic bacteria, as well as empiric fungal coverage. It is important to start broad-spectrum antimicrobial therapy because immunocompromised patients have been found to harbor unusual and resistant pathogens. The pathogens isolated are commonly polymicrobial.
In a series of 74 perianal infections in a cohort of adult patients with acute leukemia, Chen et al reported that infections were most commonly caused by gram-negative bacilli (53%), followed by gram-positive cocci (31%), anaerobes (15%), and Candida species (1%).[18] Overall, 68% of patients had polymicrobial infections, and 24% experienced bacteremia. The most commonly isolated bacteria in this study were Escherichia coli (25%), followed by Enterococcus species (22%), Klebsiella pneumonia (13%), and Bacteroides species (11%).[18] As these data show, the anorectal abscess isolates are often mixed infections, and particular attention should be given to covering enteric gram-negative organisms, such as E coli, as well as Enterococcus species.[11] The cases of bacteremia, especially with enteric pathogens, are unexpectedly low.
Our typical initial regimen dosing includes the following, adjusted for age and creatinine clearance: piperacillin/tazobactam (3.375 g IV q8hr), vancomycin (10–15 mg/kg IV q12 hr), metronidazole (500 mg IV q8hr), and diflucan (400 mg IV q8hr). The antibiotics are then tailored to pathogens once these are identified on cultures.
Because early reports of perianal disease in cancer patients showed such high mortality rates, many clinicians were concerned that surgical intervention would be associated with worse outcomes.[1,2] Shaked et al suggested that the prognosis would be better if a conservative approach was taken.[10] They found that patients could be successfully treated with supportive care and antimicrobial therapy without surgical intervention. They proposed that the ability of the patient’s neutrophil count to recover appeared to be the most important prognostic indicator. In their series of 15 patients, they reported a mortality rate of 22%, with the neutrophil count in all patients who died never recovering to > 1,500 cells/μL. In their experience, an incision and drainage procedure in a neutropenic patient did not contribute to reduction of septic episodes or mortality. Conversely, Barnes et al advocated early surgical intervention and drainage after review of their series of 16 patients. Fifteen of the sixteen neutropenic leukemia patients presenting with perianal infection either were spontaneously draining (5/16) or went to the operating room for surgical drainage (10/16). The authors suggested that early drainage preceded neutrophil count recovery of more than 500 cells/μL and that drainage was an important precursor to neutrophil recovery.[5] The only mortality in their series was a patient whose infection was not drained and who died of persistent bacteremia.
Buyukasik et al stressed the importance of the neutrophil count in the natural history of the perianal infection, and as a factor that affects choice of treatment and prognosis. Their series had a 7.3% incidence of neutropenia and perianal infection in leukemia patients, and they described the outcomes of 20 patients: 10 were surgically drained and 10 were medically managed with supportive care and antibiotics. Of the 10 patients who underwent surgical drainage, 9 experienced resolution of symptoms and 1 developed a fistula-in-ano. Of the 10 patients who were medically managed, 3 patients experienced complete resolution of the infection, 4 died as a result of sepsis, 2 developed fistulae-in-ano, and 1 patient had recurrence of infection. All patients who died (20%) were neutropenic at the time of death and all had active perianal infections.[3] The authors demonstrated that the neutrophil count during infection was an important factor affecting resolution of infection, choice of treatment, and prognosis. The median neutrophil count was higher in the operative group (1,280 cells/μL) than in the medically managed group (96 cells/μL).[5] Patients with a recovered neutrophil count were more likely to develop a defined abscess that could be drained (9 operatively vs 4 nonoperatively), while patients with persistent neutropenia typically developed nonfluctuant indurations (1 treated operatively and 6 treated nonoperatively). Of the patients with normal neutrophil counts during the infection, 10 experienced cures and 3 had complications (1 died), while of the patients with persistent neutropenia, 2 experienced cures and 5 had complications (including 3 mortalities). The ability of a patient’s neutrophil count to recover contributes to local purulence, gives rise to a need for possible drainage as the abscess becomes well defined, and improves prognosis as the patient’s immune system recovers.
A series from the National Cancer Institute involving 54 episodes of perianal infection in 44 patients with a hematologic malignancy advocated the selective use of surgical drainage. The majority of infections (55%) were resolved with supportive care and antibiotics alone. The authors reported that the most important prognostic indicator of outcome was the number of days of neutropenia during the infectious episode. They posited that surgical intervention should be reserved for patients with obvious abscess collection, significant necrosis, and progression of soft-tissue infection despite appropriate antibiotic coverage.[19] They reported a mortality of 16% with selective use of surgical drainage. A subsequent update from the same institution reported no deaths with the selective use of surgical drainage.[11] In this update of 82 episodes in 64 patients, Lehrnbecher et al reported that the majority of perianal infections (63%) were successfully treated with antimicrobial therapy only and that only about one-third (37%) required surgical drainage. Their series highlights the importance of reserving surgical intervention for patients with progressive evidence of infection or substantial fluctuance and necrosis.
Badgwell et al reported on the largest series to date of cancer patients with anorectal infection (a series of 100 patients), with the aim of identifying factors associated with surgical management.[14] This series included both patients with solid organ malignancies and those with hematologic malignancies. Forty-two patients were successfully treated nonoperatively (including 22 patients with findings consistent with an abscess) and 58 were treated operatively. Thus, even in the setting of a defined abscess, 22 patients were able to resolve their infection with antimicrobial therapy. Thrombocytopenia was associated with nonoperative management, likely due to the reluctance of the surgeon to offer drainage out of concern for bleeding. Factors associated with operative management included abscess formation and erythema on physical examination. On multivariate analysis, neutropenia was not associated with surgical intervention. The overall mortality was 1% (a patient with a necrotizing soft-tissue infection that resulted in death).
The multidisciplinary approach at our institution starts with initiating broad-spectrum antimicrobial therapy as previously described. The surgeon is then consulted, and he or she determines the extent of infection and identifies a potential abscess for drainage. A critical goal is recovery of the neutrophil count, which is an important determinant of recovery from infection. In consultation with the treating medical oncologist, the chemotherapy or immunosuppressive therapy is stopped and a colony-stimulating factor is initiated. If the neutrophil count is < 1,500 cells/μL, it is our practice to delay drainage unless there is progression of the infection or tissue necrosis. It has been our observation that patients seldom have drainable fluid if the absolute neutrophil count (ANC) is less than 500 cells/μL. Once a patient recovers from the neutropenia, he or she will often develop an abscess, and it is at that time that we would drain the collection if needed. A loose draining seton (usually a loose silastic vessel loop or a loose braided, nonabsorbable suture) is placed at the time of perianal abscess drainage if an internal opening is identified. Antimicrobial therapy is continued until there are no longer signs of infection.
Neutropenic Enterocolitis (Typhlitis or Ileocecal Syndrome)
Background
Neutropenic enterocolitis is a potentially life-threatening condition often associated with the cytotoxic chemotherapy commonly used in the treatment of acute leukemia. This condition is not to be confused with necrotizing enterocolitis, a neonatal condition characterized by bowel necrosis. Neutropenic enterocolitis is the most common diagnosis in patients presenting with the triad of: (1) neutropenia, (2) fever, and (3) abdominal pain. Other terms used for the syndrome include “typhlitis” (from the Greek word “typhlos” meaning “blind” or “closed”) and “ileocecal syndrome” (because early descriptions noted pathologic changes in the cecum and right colon).
It is important to differentiate between the different possible causes of abdominal pain in a febrile neutropenic patient so as not to delay appropriate treatment. Other causes of abdominal pain in neutropenic patients include appendicitis, cholecystitis, and cholangitis. Bowel-specific pathologies that can be similar in presentation include Clostridium difficile colitis, cytomegalovirus colitis, viral enteritis, mucositis, and graft-vs-host disease following stem cell transplant for leukemia.
Neutropenic enterocolitis has been increasingly reported in adult patients being treated with intensive cytotoxic chemotherapy agents, such as cytosine arabinoside and idarubicin.[20] The increased use of aggressive chemotherapeutic agents, such as taxanes, which cause myelosuppression and severe gastrointestinal (GI) mucositis, have also been implicated in the possible increased frequency of neutropenic enterocolitis. Patients being treated with cytotoxic chemotherapy for acute leukemia are the population most commonly affected, but neutropenic enterocolitis has also been reported in patients with lymphomas, solid organ malignancies, AIDS, aplastic anemia, and cyclic neutropenia in the absence of cytotoxic chemotherapy.[20]
Patients receiving cytotoxic chemotherapy commonly present during the third week of chemotherapy (median, 17 days), coinciding with mucosal barrier damage induced by the therapy in both hematologic and solid organ malignancies.[21] The mucosal damage caused by the cytotoxic agents gives rise to entry sites where colonizing bacteria are likely to localize and cause infection.
Incidence of and risk factors for neutropenic enterocolitis
The true incidence of neutropenic enterocolitis has been difficult to determine, given the limited number of quality studies. In the single systematic analysis of neutropenic enterocolitis in the literature, Gorschluter et al estimated the incidence in the adult population to be 5.3% in patients hospitalized for hematologic malignancies, for high-dose chemotherapy for solid tumors, or for aplastic anemia.[22] As noted above, the incidence seems to be increasing with the increased use of aggressive cytotoxic chemotherapy regimens that result in neutropenia and mucositis.
Pathologic, clinical, and radiologic criteria for neutropenic enterocolitis have not been clearly defined, and some authors have questioned whether neutropenic enterocolitis is actually a distinct entity. The ambiguity surrounding the inclusion of patients in case series and reports makes it difficult to determine whether all patients actually had neutropenic enterocolitis or whether they were misclassified and had other conditions, such as mucositis, graft-vs-host disease, or C difficile colitis. Abdominal imaging with either ultrasound or CT to evaluate for bowel wall thickness (BWT) is now recognized as playing an important role in accurate diagnosis.[20,22,23] To make the diagnosis of neutropenic enterocolitis objective, measurable, and reproducible, more recent authors have offered a refined definition that includes the triad of neutropenia (< 500 cells/μL), fever (> 38.3°C, rectal or oral), and abdominal pain, combined with imaging (ultrasound or CT) demonstrating BWT > 4 mm in a segment of bowel at least 3 cm in length.[20,22,23] Still, the lack of consensus regarding the etiology and definition of neutropenic enterocolitis has made accurate calculations of incidence challenging.
Patients with neutropenic enterocolitis usually share a common characteristic of having undergone cytotoxic chemotherapy, with the initial reports involving primarily cytosine arabinoside and idarubicin.[24,25] More recent reports demonstrate an association of neutropenic enterocolitis with taxanes (docetaxel and paclitaxel) and vinorelbine used in the treatment of various solid organ malignancies, including ovarian, lung, and breast cancers.[24-26] Many of these chemotherapeutic agents have been associated with severe mucositis. Other experts have reported patients with neutropenic enterocolitis after treatment with fluorouracil, capecitabine, cisplatin, carboplatin, cyclophosphamide, and ifosfamide.[27-30]
The degree and severity of neutropenic enterocolitis is associated with the degree of neutropenia (< 100 cells/μL) and its duration.[20] In a report of pediatric patients with cancer, mucositis (odds ratio [OR], 31), hematopoietic stem cell transplantation (OR, 59), and receipt of chemotherapy in the previous 2 weeks (OR, 13) were significantly associated with the occurrence of neutropenic enterocolitis.[31] The morbidity and mortality associated with neutropenic enterocolitis can be significant, with mortalities as high as 50% to 100%.[32,33]
Pathogenesis and microbiology of neutropenic enterocolitis
The pathogenesis of neutropenic enterocolitis is incompletely understood. It likely involves the combination of mucosal injury induced by cytotoxic chemotherapy or radiation, immunosuppression from severe neutropenia, and impaired host defense against commensal enteric microorganisms. The result is necrosis of various layers of the bowel wall. The location of intestinal inflammation with bowel wall thickening and pneumatosis was initially identified as being in the cecum. This inflammatory state of the submucosa results in the clinical symptoms of the syndrome. Nuclear factor–kappa B and other proinflammatory cytokines contribute to epithelial cell apoptosis. Microscopic specimens have nonpathognomonic features of mucosal and submucosal edema, hemorrhage, and necrosis.
The cecum is thought to be frequently affected because of its large circumference, distensibility, and potentially decreased blood supply. The inflammation and bowel wall thickening are not exclusive to the cecum, however, and have been shown to affect other areas of the small and large bowel. In fact, neutropenic enterocolitis has been found to involve the cecum alone in 28% of cases, with extensive colonic involvement in 75% of cases and small bowel involvement in 66%.[23]
Neutropenic enterocolitis is often a polymicrobial infection involving gram-negative bacilli, gram-positive cocci, anaerobes, and Candida species. Enteric organisms such as Pseudomonas aeruginosa, E coli, Klebsiella species, viridans streptococci, the enterococci, and anaerobes such as Bacteroides species and Clostridium species were the most frequently isolated organisms based on microbiologic cultures obtained from peritoneal ascites and bowel wall specimens.[20] Candida species are the most common cause of fungal bloodstream infection in patients with neutropenic enterocolitis, but Candida infection is much less common than bacterial infection.
Diagnosis and imaging characteristics of neutropenic enterocolitis
The diagnosis of neutropenic enterocolitis should be considered in any severely neutropenic patient (ANC < 500 cells/μL) who presents with fever > 38.3°C (oral or rectal), abdominal pain, cramping, distention, diarrhea, or lower GI bleeding. Many other abdominal etiologies with similar signs and symptoms should also be considered in a febrile neutropenic patient.
To increase the sensitivity of the diagnosis of neutropenic enterocolitis, the clinical features of fever, neutropenia, and abdominal pain should be combined with radiologic studies. The main imaging modalities used in patients in whom neutropenic enterocolitis is suspected include plain films, ultrasound, and CT. Plain films lack sensitivity and specificity for the diagnosis. However, findings of thumbprinting of the thickened bowel or a lack of air in the right lower quadrant are supportive of the diagnosis. Free air found on plain films supports a diagnosis of perforation, and in such cases surgical consultation should not be delayed. Ultrasound has the advantage of being able to be performed at the bedside in patients who cannot be transported to the radiology suite, along with the benefit of no ionizing radiation exposure. Ultrasound images will often show thickening of the bowel (> 4 mm), increased density in the center of the bowel, and a wide hypoechoic periphery.
CT is the imaging study of choice to differentiate neutropenic enterocolitis from other possible causes of pain in a neutropenic patient presenting with fever and abdominal pain. Kirkpatrick et al retrospectively reviewed 76 patients who presented with neutropenia, fever, and abdominal pain. Based on the characteristics of the bowel thickness and mesenteric characteristics, they were able to formulate CT diagnostic criteria that could differentiate between neutropenic enterocolitis, graft-vs-host disease, and C difficile colitis. On imaging, neutropenic enterocolitis has thickened bowel-on average 7 mm thick (range, 4–15 mm) and is associated with mesenteric stranding (Figure 2A). Graft-vs-host disease has minimal bowel wall thickening but significant mucosal enhancement (Figure 2B). C difficile colitis has prominent bowel wall thickening (> 12 mm) and wall nodularity (Figure 2C). By using the combination of the clinical features and CT bowel thickening of > 4 mm, the diagnosis of neutropenic enterocolitis can be more objective, reproducible, and measurable.[23]
Management of neutropenic enterocolitis
All neutropenic patients who present with fever and abdominal pain should have a complete blood cell count, complete metabolic panel, and coagulopathy studies performed. Blood cultures should be performed as well-at least two sets and a set from each lumen of an indwelling blood catheter. C difficile testing with toxin assay or polymerase chain reaction and stool cultures to rule out other causes of colitis should be completed. In patients in whom neutropenic enterocolitis is suspected, both barium enemas and colonoscopic examinations are relatively contraindicated, given the increased risk of perforation.
Neutropenic enterocolitis was originally thought to be a terminal complication of treatment for acute leukemia, with very high morbidity and mortality.[34,35] Early reports by Varki et al, in 1979, documented the successful treatment of neutropenic enterocolitis with surgical resection, including a right hemicolectomy and ileocolonic anastomosis in a patient with acute lymphoblastic leukemia.[36] This approach was advocated based on the concept that the natural history of neutropenic enterocolitis was progression to full-thickness inflammation and edema, resulting in hemorrhage, necrosis, and perforation of the bowel. Because of improvements in supportive care, more recent authors report successful management without surgical intervention. This nonoperative management includes bowel rest with nothing by mouth, intravenous fluid support, parenteral nutrition if needed, nasogastric tube suction if needed, broad-spectrum antibiotics, and granulocyte-macrophage colony-stimulating factor (GM-CSF). Platelet transfusions may be necessary for severe thrombocytopenia, and coagulopathies should be corrected. GM-CSF can be used in select patients and is thought to hasten the recovery of the neutrophil count, but its use has not been specifically studied in this population.
The initial choice of antibiotics should include agents with gram-negative, gram-positive, and anaerobic coverage, but the choice should reflect local epidemiology, susceptibility or resistance patterns, and previous antimicrobial exposure. Monotherapy with agents such as imipenem, meropenem, or piperacillin/tazobactam may be adequate in some patients, but combination therapy should be used in patients known to be colonized with, or suspected of having, an infection with a resistant pathogen (extended-spectrum β-lactamase–producing gram-negative bacilli, vancomycin-resistant enterococci, methicillin-resistant Staphylococcus aureus, or a multidrug-resistant organism). Antifungal coverage is not routinely recommended, since the incidence of fungal-associated neutropenic enterocolitis is only about 5%.[37] It is reasonable to add antifungal coverage (fluconazole, 400 mg IV qd, with renal adjustments) if 72–96 hours of antibiotic coverage fails to produce signs of clinical improvement. Blood cultures, while positive in only 18% to 44% of patients, can be used to guide therapy.[24] Antimicrobial therapy should be continued until body temperature has normalized, neutrophil and platelet counts (the latter in instances of thrombocytopenia) have recovered, bacteremia has resolved, and normal GI function has returned. Further imaging should be performed if symptoms persist despite full supportive care. Antineoplastic regimens should be delayed until full recovery from neutropenic enterocolitis. Subsequent chemotherapy treatment should avoid the offending agent/regimen.
Surgical intervention is now reserved for select cases, as outlined by Shamberger et al. These authors recommend that surgery be reserved for patients who have (1) persistence of GI bleeding despite correction of coagulopathies, thrombocytopenia, and neutropenia; (2) free air in the intraperitoneal cavity indicative of bowel perforation; (3) clinical deterioration despite optimal medical management; or (4) the development of other indications for surgery, such as appendicitis.[38] A conservative management strategy is advocated unless one of these criteria is present, and it may be best, if possible, to delay surgical intervention until the neutrophil count improves.[39]
Treatment of Appendicitis in the Neutropenic Cancer Patient
Chemotherapy-induced neutropenia increases a patient’s susceptibility to a range of serious infectious complications. GI infections account for nearly 30% of these infections.[40] Acute appendicitis occurs in 1.5% of pediatric patients treated with chemotherapy for leukemia or lymphoma[41]; the incidence in the adult population is unknown. The diagnosis of acute appendicitis is not always straightforward in cancer patients, as neutropenic enterocolitis can have a similar presentation and imaging findings. CT in a patient who presents with fever, right lower abdominal pain, and neutropenia is important in determining the cause of the infection. If the inflammation is limited to the appendix (and does not involve the adjacent cecum or ascending colon), and if the diagnostic CT findings of inflammation, thickening and enhancement of the appendiceal wall, and periappendiceal fat stranding are present, then the clinician can feel confident of the diagnosis of acute appendicitis. Once the diagnosis is made, the clinician must weigh the risks and benefits of surgical intervention.
Uncomplicated appendicitis in a neutropenic patient in the era of minimally invasive surgery is best treated with laparoscopic appendectomy. Mortellaro et al reported their experience of treating 11 neutropenic pediatric patients diagnosed with acute appendicitis, with a follow-up of nearly 2 years. Appendectomy was performed successfully without complications, resulting in a postoperative length of stay of 4 days and early return to enteral feeding.[42] None of the patients experienced enteric fistula or wound infections from the interventions. These results confirm in a small cohort that appendectomy can be performed with success and efficiency. Wiegering et al have advocated nonoperative management and reported their findings of 5 patients treated with nothing by mouth, antibiotics, and intravenous fluids. Their nonoperative management strategy required a prolonged course of antibiotics and longer periods of analgesics. In addition, more than 50% of the patients had recurrent symptoms of right lower quadrant pain on subsequent rounds of chemotherapy.[43]
Uncomplicated appendicitis in a neutropenic patient is probably best treated with appendectomy; however, complicated appendicitis is likely best treated with supportive care, antibiotics, and possibly CT-guided drainage if the situation is amenable to this. Source control of infection in a neutropenic patient is paramount (at the appendectomy site or drain), and supportive care with broad-spectrum antibiotics is our preferred management strategy in cases where there are complications.
Treatment of Cholecystitis in the Neutropenic Cancer Patient
Acute cholecystitis is uncommon in neutropenic cancer patients. The estimated incidence is 0.4%, and this low rate is likely due to the small mucosal surface area of the gallbladder. The diagnosis is made based on right upper quadrant ultrasound findings. The classic findings include a gallbladder wall thickness of 3 mm, pericholecystic fluid, striated thickening of the gallbladder wall, biliary sludge, and overdistention. Neutropenic patients in whom acute cholecystitis is diagnosed are more likely to have acalculous cholecystitis (67%) than are nonneutropenic patients (5%). Neutropenic patients in whom acute cholecystitis is diagnosed have a high mortality rate (26%), but only 14% of these deaths have been primarily caused by complications of the cholecystitis.[44]
Surgical intervention has been associated with a nearly 50% mortality rate and should be reserved for gangrenous cholecystitis or perforation that less invasive methods would not benefit. For the majority of patients, a less invasive strategy is preferred; this includes supportive care with intravenous fluids, broad-spectrum antibiotics, and percutaneous drainage. Many experts advocate placement of an image-guided cholecystostomy tube for acute resolution of inflammation and drainage. This allows for source control, improvement in symptoms, and time to improve the neutropenia. A minimally invasive cholecystectomy may then be performed electively.
Financial Disclosure:The authors have no significant financial interest in or other relationship with the manufacturer of any product or provider of any service mentioned in this article.
References:
1. Musa MB, Katakkar SB, Khaliq A. Anorectal and perianal complications of hematologic malignant neoplasms. Can J Surg. 1975;18:579-83.
2. Schimpff SC, Wiernik PH, Block JB. Rectal abscesses in cancer patients. Lancet. 1972;2:844-7.
3. Buyukasik Y, Ozcebe OI, Sayinalp N, et al. Perianal infections in patients with leukemia: importance of the course of neutrophil count. Dis Colon Rectum. 1998;41:81-5.
4. Abcarian H. Anorectal infection: abscess-fistula. Clin Colon Rectal Surg. 2011;24:14-21.
5. Barnes SG, Sattler FR, Ballard JO. Perirectal infections in acute leukemia. Improved survival after incision and debridement. Ann Intern Med. 1984;100:515-8.
6. Grewal H, Guillem JG, Quan SH, et al. Anorectal disease in neutropenic leukemic patients. Operative vs. nonoperative management. Dis Colon Rectum. 1994;37:1095-9.
7. Vanhueverzwyn R, Delannoy A, Michaux JL, Dive C. Anal lesions in hematologic diseases. Dis Colon Rectum. 1980;23:310-2.
8. Corfitsen MT, Hansen CP, Christensen TH, Kaae HH. Anorectal abscesses in immunosuppressed patients. Eur J Surg.1992;158:51-3.
9. Morcos B, Amarin R, Abu Sba A, et al. Contemporary management of perianal conditions in febrile neutropenic patients. Eur J Surg Oncol. 2013;39:404-7.
10. Shaked AA, Shinar E, Freund H. Managing the granulocytopenic patient with acute perianal inflammatory disease. Am J Surg. 1986;152:510-2.
11. Lehrnbecher T, Marshall D, Gao C, Chanock SJ. A second look at anorectal infections in cancer patients in a large cancer institute: the success of early intervention with antibiotics and surgery. Infection. 2002;30:272-6.
12. Rizzo JA, Naig AL, Johnson EK. Anorectal abscess and fistula-in-ano: evidence-based management. Surg Clin North Am. 2010;90:45-68, Table of Contents.
13. Ramanujam PS, Prasad ML, Abcarian H, Tan AB. Perianal abscesses and fistulas. A study of 1023 patients. Dis Colon Rectum. 1984;27:593-7.
14. Badgwell BD, Chang GJ, Rodriguez-Bigas MA, et al. Management and outcomes of anorectal infection in the cancer patient. Ann Surg Oncol. 2009;16:2752-8.
15. Morris J, Spencer JA, Ambrose NS. MR imaging classification of perianal fistulas and its implications for patient management. Radiographics. 2000;20:623-35; discussion 35-7.
16. Buchanan GN, Halligan S, Bartram CI, et al. Clinical examination, endosonography, and MR imaging in preoperative assessment of fistula in ano: comparison with outcome-based reference standard. Radiology. 2004;233:674-81.
17. Baker B, Al-Salman M, Daoud F. Management of acute perianal sepsis in neutropenic patients with hematological malignancy. Tech Coloproctol. 2014;18:327-33.
18. Chen CY, Cheng A, Huang SY, et al. Clinical and microbiological characteristics of perianal infections in adult patients with acute leukemia. PLoS One. 2013;8:e60624.
19. Glenn J, Cotton D, Wesley R, Pizzo P. Anorectal infections in patients with malignant diseases. Rev Infect Dis. 1988;10:42-52.
20. Nesher L, Rolston KV. Neutropenic enterocolitis, a growing concern in the era of widespread use of aggressive chemotherapy. Clin Infect Dis. 2013;56:711-7.
21. Bow EJ, Meddings JB. Intestinal mucosal dysfunction and infection during remission-induction therapy for acute myeloid leukaemia. Leukemia. 2006;20:2087-92.
22. Gorschluter M, Mey U, Strehl J, et al. Neutropenic enterocolitis in adults: systematic analysis of evidence quality. Eur J Haematol. 2005;75:1-13.
23. Kirkpatrick ID, Greenberg HM. Gastrointestinal complications in the neutropenic patient: characterization and differentiation with abdominal CT. Radiology. 2003;226:668-74.
24. Gomez L, Martino R, Rolston KV. Neutropenic enterocolitis: spectrum of the disease and comparison of definite and possible cases. Clin Infect Dis. 1998;27:695-9.
25. Gorschluter M, Marklein G, Hofling K, et al. Abdominal infections in patients with acute leukaemia: a prospective study applying ultrasonography and microbiology. Br J Haematol. 2002;117:351-8.
26. Aksoy DY, Tanriover MD, Uzun O, et al. Diarrhea in neutropenic patients: a prospective cohort study with emphasis on neutropenic enterocolitis. Ann Oncol. 2007;18:183-9.
27. Bremer CT, Monahan BP. Necrotizing enterocolitis in neutropenia and chemotherapy: a clinical update and old lessons relearned. Curr Gastroenterol Rep. 2006;8:333-41.
28. Gordon VL, Harding GA, Czaykowski P. Capecitabine-induced, nonneutropenic enterocolitis. J Gastrointest Cancer. 2011;42:278-81.
29. Kronawitter U, Kemeny NE, Blumgart L. Neutropenic enterocolitis in a patient with colorectal carcinoma: unusual course after treatment with 5-fluorouracil and leucovorin-a case report. Cancer. 1997;80:656-60.
30. Vlasveld LT, Ten Bokkel Huinink WW, Rodenhuis S. Neutropenic enterocolitis in a patient with ovarian cancer after treatment with high-dose carboplatin and granulocyte macrophage-colony stimulating factor (GM-CSF). Neth J Med. 1990;37:158-61.
31. Moran H, Yaniv I, Ashkenazi S, et al. Risk factors for typhlitis in pediatric patients with cancer. J Pediatr Hematol Oncol. 2009;31:630-4.
32. Alt B, Glass NR, Sollinger H. Neutropenic enterocolitis in adults. Review of the literature and assessment of surgical intervention. Am J Surg. 1985;149:405-8.
33. Wade DS, Nava HR, Douglass HO, Jr. Neutropenic enterocolitis. Clinical diagnosis and treatment. Cancer. 1992;69:17-23.
34. Katz JA, Wagner ML, Gresik MV, et al. Typhlitis. An 18-year experience and postmortem review. Cancer. 1990;65:1041-7.
35. Sloas MM, Flynn PM, Kaste SC, Patrick CC. Typhlitis in children with cancer: a 30-year experience. Clin Infect Dis. 1993;17:484-90.
36. Varki AP, Armitage JO, Feagler JR. Typhlitis in acute leukemia: successful treatment by early surgical intervention. Cancer. 1979;43:695-7.
37. Gorschluter M, Mey U, Strehl J, et al. Invasive fungal infections in neutropenic enterocolitis: a systematic analysis of pathogens, incidence, treatment and mortality in adult patients. BMC Infect Dis. 2006;6:35.
38. Shamberger RC, Weinstein HJ, Delorey MJ, Levey RH. The medical and surgical management of typhlitis in children with acute nonlymphocytic (myelogenous) leukemia. Cancer. 1986;57:603-9.
39. Badgwell BD, Cormier JN, Wray CJ, et al. Challenges in surgical management of abdominal pain in the neutropenic cancer patient. Ann Surg. 2008;248:104-9.
40. Koretz MJ, Neifeld JP. Emergency surgical treatment for patients with acute leukemia. Surg Gynecol Obstet. 1985;161:149-51.
41. Hobson MJ, Carney DE, Molik KA, et al. Appendicitis in childhood hematologic malignancies: analysis and comparison with typhlitis. J Pediatr Surg. 2005;40:214-9; discussion 19-20.
42. Mortellaro VE, Juang D, Fike FB, et al. Treatment of appendicitis in neutropenic children. J Surg Res. 2011;170:14-6.
43. Wiegering VA, Kellenberger CJ, Bodmer N, et al. Conservative management of acute appendicitis in children with hematologic malignancies during chemotherapy-induced neutropenia. J Pediatr Hematol Oncol. 2008;30:464-7.
44. Gorschluter M, Mey U, Strehl J, et al. Cholecystitis in neutropenic patients: retrospective study and systematic review. Leuk Res. 2006;30:521-8.
Late Hepatic Recurrence From Granulosa Cell Tumor: A Case Report
Granulosa cell tumors exhibit late recurrence and rare hepatic metastasis, emphasizing the need for lifelong surveillance in affected patients.