Treatment of Peripheral T-Cell Lymphoma: Many Shades of Gray
This article evaluates the most up-to-date peer-reviewed published work on the treatment of peripheral T-cell lymphoma, and offers a glimpse into the current upfront clinical trial landscape for patients affected by this uncommon disease.
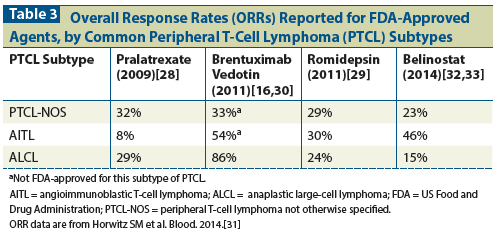
Table 1: Ongoing Phase III Studies in Peripheral T-Cell Lymphoma
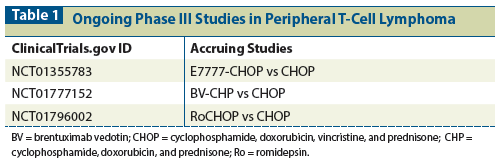
Table 2: Selected Reports With Data on Upfront Consolidative Autologous Stem Cell Transplant in Peripheral T-Cell Lymphoma
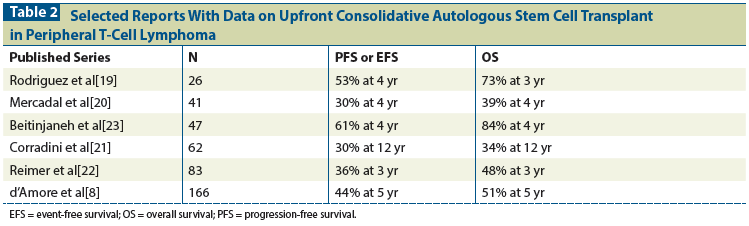
Table 3: Overall Response Rates (ORRs) Reported for FDA-Approved Agents, by Common Peripheral T-Cell Lymphoma (PTCL) Subtypes
Previously obscured within other designations of aggressive lymphomas, peripheral T-cell lymphoma (PTCL) now represents 23 different subtypes of non-Hodgkin lymphoma (NHL). Despite the many subtypes now recognized, PTCL represents only approximately 10% of all NHL cases diagnosed. Positron emission tomography/computed tomography has become essential to accurate staging and response-evaluation for PTCL. In comparison to aggressive B-cell NHL, patients with PTCL will more often be refractory to initial therapy, and chemosensitive patients will have shorter disease-free periods. Anthracycline-based regimens, often with the inclusion of etoposide, are commonly used during induction therapy. Consolidation with high-dose therapy and autologous stem cell transplantation (ASCT) in first chemosensitive remission appears to provide the best outcome in common nodal PTCL subtypes. The commonly defined nodal subtypes are PTCL not otherwise specified, angioimmunoblastic T-cell lymphoma, and anaplastic lymphoma kinase (ALK)-positive or ALK-negative anaplastic large-cell lymphoma (ALCL). Four agents have been approved by the US Food and Drug Administration for use in the relapsed/refractory (rel/ref) setting, including belinostat (2014), romidepsin (2011), brentuximab vedotin (2011), and pralatrexate (2009). Brentuximab vedotin was approved only for the ALCL subtype. These agents continue to be studied as combinations in the rel/ref setting and as additions or substitutions for other agents in upfront multiagent chemotherapy regimens. Patients who have responded to treatment in the rel/ref setting and are considered transplant-eligible should be considered for allogeneic stem cell transplantation, especially those with previous ASCT. Upfront allogeneic stem cell transplantation remains a research question in the majority of PTCL subtypes, but data are emerging.
Introduction
Twenty-three different variations of peripheral T-cell lymphoma (PTCL) have been defined by the 2008 World Health Organization classification.[1] There have been significant strides in the attempt to improve the diagnostic accuracy of the approximately 6,000 cases of PTCL diagnosed each year in the United States.
Diagnostic accuracy has been enhanced by the use of expanded immunohistochemistry assessment, occasional pathognomonic cytogenetic results, and molecular studies. However, molecular evaluations other than T-cell receptor clonality studies have yet to translate to significant clinical application. With increased availability of positron emission tomography/computed tomography (PET/CT), the staging or extent-of-disease evaluation for patients with PTCL has become more essential and reliable, leaving localized or early-stage PTCL as an uncommon clinical scenario without a definitive standard of care. Once a diagnosis of PTCL is rendered, the stage is confirmed, and prognostic index variables are documented, an initial treatment regimen must be chosen.[2,3] Although the management landscape in PTCL is rapidly evolving, a few aspects are generally agreed upon: (1) a diagnosis of systemic PTCL (excluding cutaneous T-cell lymphoma) requires multiagent chemotherapy in order to be considered on a curative pathway; (2) clinical trial enrollment is paramount at any phase of care; and (3) in under a decade, four agents have been approved by the US Food and Drug Administration (FDA) for rel/ref PTCL. Beyond these basic agreements, the approach to diagnosis becomes more opaque, clinical outcomes are often statistically underpowered, case reports become more common, and prospective studies are scarce-that is, the grayscale of care becomes visible. This article evaluates the most up-to-date peer-reviewed published work on the treatment of PTCL, and offers a glimpse into the current upfront clinical trial landscape for patients affected by this uncommon disease-one that, it is hoped, will usher in a new or expanded era of management in PTCL.
The Future of Induction Therapy in PTCL: You’ve Been CHOPped
Progress in the treatment of PTCL has been gaining momentum, but breaking away from models of aggressive B-cell lymphoma has been a challenge. It is acknowledged that CHOP (cyclophosphamide, doxorubicin, vincristine, and prednisone) was borrowed from the treatment of B-cell non-Hodgkin lymphoma (NHL) as an option for induction therapy in patients with PTCL.[4] However, historic data in PTCL remain relevant compared with the majority of the data that preceded the rituximab era in the management of B-cell NHL. A large prospective trial of CHOP alone as induction therapy is not available in PTCL outside of an adult T-cell lymphoma/leukemia (ATLL) study in Japan.[5] To set the stage in PTCL, the BC [British Columbia] Cancer Agency (BCCA) and German High-Grade Non-Hodgkin Lymphoma Study Group (DSHNHL) retrospective series provided a baseline against which to measure outcomes in PTCL going forward.[6,7] The BCCA report on 191 patients with PTCL demonstrated that patients with anaplastic lymphoma kinase (ALK)-negative anaplastic large-cell lymphoma (ALCL) and angioimmunoblastic T-cell lymphoma (AITL) had similar 5-year overall survival (OS) rates of 34% and 36%, respectively.
In the peripheral T-cell lymphoma not otherwise specified (PTCL-NOS) cohort, the 5-year progression-free survival (PFS) and OS rates were 29% and 35%, respectively. The second series completed by the DSHNHL included 320 patients with PTCL from eight prospective trials. Adults < 60 years of age and those with a normal lactate dehydrogenase level at presentation had a worse outcome with CHOP alone vs CHOP plus etoposide (CHOEP). Of note, ALCL patients with the ALK-positive subtype benefited most from addition of etoposide. CHOEP was felt to be too toxic for patients over age 60, and its use should be considered on a case-by-case basis for this age group. These studies serve as historic baselines for future comparison of therapies in this setting.
Subsequently, research by the Nordic Lymphoma Group ushered in an era of prospective data with CHOEP in patients less than 60 years of age for whom consolidation with autologous stem cell transplantation (ASCT) was planned.[8] Treatment with CHOEP yielded a complete response (CR) in 63% of patients and an overall response rate (ORR) of 82% by CT-based response criteria. PET/CT was not used to determine response in this study. Arguably, given these results it can be concluded that CHOEP is an efficacious approach, particularly in patients younger than 60 years of age who are considering a consolidation transplant approach. CHOP remains an option for patients who are over age 60 or believed to be unable to tolerate CHOEP.
Use of dose-adjusted (DA)-EPOCH (etoposide, cyclophosphamide, doxorubicin, vincristine, and prednisone), a continuous infusion–based regimen, is an option based on National Comprehensive Cancer Network (NCCN) guidelines. Results of DA-EPOCH in the upfront setting in common PTCL subtypes have yet to be published in a peer-reviewed journal, but a significant body of clinical experience and research is available in the medical literature regarding its use in aggressive B-cell NHL.[9] The economic impact of DA-EPOCH is likely to be greater than that of CHOP/CHOEP in PTCL, as many institutions still require a 5-day inpatient stay for completion of the continuous-
infusion protocol.
Alemtuzumab, a CD52 antibody, plus CHOP (AL-CHOP) has been found to yield a high ORR of 90% but a short median event-free survival time of 10 months. However, significant concerns have been expressed regarding the risk of infectious death with this regimen.[10] Furthermore, Corradini et al reported an age-adapted approach with alemtuzumab (30 mg for patients younger than 60 years of age; 10 mg for patients 60 years of age or older) in combination with CHOP. In patients 60 years or older, the intent was to administer 6 cycles of AL-CHOP. Among the patients evaluable for response, the CR rate was 72% with a 4-year OS of 31%.[11] Alemtuzumab has also been studied as a maintenance approach post induction therapy with CHOEP, where etoposide was excluded for patients over age 60 by the DSHNHL group.[12] A CR or very good partial response (VGPR) to CHOEP was seen in 33 of 41 patients (80.5%). (VGPR is not a recognized response criterion in the recently updated Lugano Classification response criteria.[13]) A total of 29 of 41 patients went on to receive alemtuzumab maintenance. Interestingly, the 3-year OS rate among those treated with alemtuzumab was 75%, and most patients received the intended doses. In the alemtuzumab cohort, significant infectious toxicities were noted, and possibly one treatment-related death. The authors concluded that despite the results reported, the maintenance concept has been abandoned in favor of inclusion of alemtuzumab during induction therapy to possibly prevent early progression in patients over age 60. The younger cohort (patients < 60 years old) received only 2 cycles of AL-CHOP prior to transition to a higher-intensity regimen and subsequently ASCT or allogeneic stem cell transplant, based on donor availability. The ORR was 62% in this cohort. These results do not appear to be practice-changing, especially in the cohort of patients over 60 years of age. The use of alemtuzumab in PTCL has not gained much traction in the induction or rel/ref setting in North America.
In a retrospective fashion, the International Peripheral T-Cell Lymphoma Project reported the outcomes of 1,314 patients with PTCL. Interestingly, patients treated with anthracycline-containing regimens did not necessarily have better outcomes compared with those who received non-anthracycline regimens.[2] This series did not discuss why certain patients did not receive an anthracycline-based regimen. Given these results and the unsatisfactory results with CHOP alone, non-anthracycline regimens have been explored. The first prospective study of a non-anthracycline regimen evaluated the PEGS regimen (cisplatin, etoposide, gemcitabine, and methylprednisolone).[14] Among newly diagnosed patients only (as the study allowed relapsed patients later), the PEGS regimen demonstrated an ORR of 39% and a 2-year PFS rate of 14%. In the United Kingdom, CHEMO-T (ClinicalTrials.gov ID: NCT01719835), a phase II study, is using a similar non-anthracycline combination with cisplatin, gemcitabine, and methylprednisolone (GEM-P); study results have not yet been reported. Lastly, the non–anthracycline-based regimen CEOP (cyclophosphamide, etoposide, vincristine, and prednisone), alternating with pralatrexate (P), was tested in 33 patients in a phase II study by the North American Peripheral T-Cell Lymphoma Consortium; in their most recent update of the data, CEOP-P resulted in a CR rate of 52%, an ORR of 70%, and 64% OS at 2 years.[15] Unfortunately, the prespecified endpoint was not met in this study, and it is uncertain whether this regimen will be assessed further in a randomized phase III study.
Accrual into new studies continues, in an attempt to improve outcomes by combining CHOP with novel agents, despite noncomparative prospective data on combination therapy including CHOEP. The use of novel agents in combination with CHOEP is also likely to be explored (Table 1). Brentuximab vedotin, a monomethyl auristatin E (MMAE) antibody conjugate directed at CD30 that is approved for the treatment of rel/ref ALCL, is the first novel agent for which early data on combination treatment for PTCL are available.[16] In addition, a phase I study evaluated brentuximab vedotin combined with cyclophosphamide, doxorubicin, and prednisone (CHP) in newly diagnosed patients with CD30-positive PTCL; the reported CR rate was 88% and the estimated 1-year PFS rate was 71%.[17] As a result of these strong findings, brentuximab vedotin plus CHP has bypassed traditional phase II testing and is the subject of a randomized phase III study (ClinicalTrials.gov ID: NCT01777152) in newly diagnosed CD30-expressing PTCL, with the control arm being CHOP.
Consolidative Autologous Transplant: Is It a De Facto Standard?
Regardless of the results with induction therapy alone in PTCL, we have not reached disease-free survival rates comparable to those achieved in patients with other aggressive B-cell NHLs. To date, randomized prospective clinical trials evaluating ASCT vs expectant observation in first remission have only been carried out in patients with aggressive B-cell NHLs, with no benefit seen for consolidation in the setting of B-cell NHLs.[18] For patients with PTCL, however, patient assessment for possible consolidation therapy remains a common reason for consultation at regional transplant centers.
While retrospective and prospective data on consolidation therapy in this setting continue to be impressive (Table 2), the availability of recently approved novel agents may change the treatment paradigm in PTCL, namely the management of ALK-negative ALCL.[8,19-23] Naturally, the more common nodal subtypes of PTCL-PTCL-NOS, AITL, and ALK-negative ALCL-are disproportionately represented in the literature. A notable exclusion is ALK-positive ALCL, as upfront ASCT studies have generally excluded these patients, given the excellent disease-free survival.[24]
The most noteworthy trial to date remains the intent-to-transplant study by the Nordic Lymphoma Group, which assigned patients with chemosensitive response to CHOEP by CT criteria (CR or PR) to a BEAM (carmustine, etoposide, cytarabine, melphalan)-based ASCT.[8] By intent to treat/transplant, 72% proceeded to ASCT; overall, 5-year PFS and OS rates were 51% and 44%, respectively. Those with PTCL-NOS and AITL had a 5-year PFS of 38% and 49% and OS of 49% and 47%, respectively. Patients with ALK-negative ALCL fared best, with 5-year PFS and OS of 61% and 70%, respectively.
Emerging adjuncts to the upfront transplant approach are being encountered in the clinic as further data and newer therapeutic agents become available. In both retrospective and prospective studies, the results of patients with PTCL-NOS and AITL treated with upfront transplant denote an ongoing opportunity for possible post-transplant maintenance approaches, given their respective PFS outcomes. An example would be the currently accruing lenalidomide maintenance trial (ClinicalTrials.gov ID: NCT01035463). To date, maintenance therapy remains within the realm of clinical trials. Another consideration would be to acknowledge the excellent ORR of 86% observed with brentuximab vedotin in the rel/ref setting in ALK-negative or ALCL-positive disease. Therefore, an attractive approach may be to delay any transplant until after exposure to brentuximab vedotin in the second-line setting. This would allow for delaying the patient-specific risk of transplant-related morbidity/mortality; it carries a potential cost savings, albeit one for which there are no comparative prospective data. The Center for International Blood and Marrow Transplant Research recently published data that would support this concept specifically in ALCL.[25] Outside of common nodal subtypes, use of upfront ASCT is guided more by benefit seen in limited subtype analysis in previously reported transplant studies (ie, enteropathy-associated T-cell lymphoma) and extrapolation from single-institution or retrospective case series involving treatment of less common PTCL subtypes.
Relapsed/Refractory PTCL: Are We Helping More Patients?
Multiagent chemotherapy continues to be the standard of care for upfront or induction treatment of PTCL. Unfortunately, even those who obtain the best response-a PET/CT-negative CR-are still at a high risk for relapse. Therefore, strategies for the rel/ref patient with PTCL often have had to diverge from the aggressive B-cell NHL model, where intensive second-line chemotherapy followed by ASCT is the standard of care. Standard more intensive combination therapies are often employed in patients who are planning or proceeding towards a consolidative allogeneic stem cell transplant, or in those who have had a long disease-free interval after initial therapy. Experiences with both ICE (ifosphamide, carboplatin, and etoposide) and bendamustine have reflected relatively high ORRs compared with the other approved agents, but short PFS.[26,27] For instance, patients treated with bendamustine had an ORR of 50% but just a 3.5-month duration of response.
Therefore, in rel/ref PTCL, the paradigm has shifted significantly towards potentially milder single-agent treatments that can be considered for multiple cycles in a continuous fashion; this approach has yielded similar ORRs between agents, but patient response often differs by PTCL subtype (Table 3). This paradigm shift has resulted in approval of four single agents. In the phase II study (PROPEL) that led to the first FDA-approved drug in PTCL, 111 patients with rel/ref PTCL received pralatrexate, an intravenous drug given for 6 weeks in 7-week cycles.[28] The CR rate was 11% and the ORR was 29%. Interestingly, subtype divergence by ORR was seen, as patients with AITL had an ORR of only 8%, but those with PTCL-NOS had an ORR of 32%. In the 29% of patients who achieved a response, the median duration of remission was 10 months. The most significant toxicity associated with pralatrexate was oral mucositis, but this adverse event can often be avoided by alteration of the administration schedule.
Romidepsin was the second drug approved for rel/ref PTCL. In two phase II studies, romidepsin yielded an ORR of 38% in patients with either cutaneous lymphomas or PTCL, and 25% in those with only PTCL.[29] The median duration of response was 17 months. Compared with the 8% ORR with pralatrexate in AITL, romidepsin yielded an ORR of 30%. The main side effects associated with romidepsin were fatigue, nausea, and thrombocytopenia. Magnesium and potassium were optimized in the phase II study; however, no language about electrolyte imbalance was included in the packaging label, and outside of a clinical trial, correction of electrolyte levels remains at the discretion of the administering physician.
In ALCL, CD30 overexpression is the hallmark immunophenotypic aberration. CD30 expression can be seen to a lesser extent in other subtypes of PTCL. Brentuximab vedotin was initially studied in rel/ref ALCL. In a phase II study, it yielded an impressive ORR of 86%, with a CR rate of 57% in both ALK-positive and ALK-negative patients; these results led to its approval for management of ALCL.[16] The drug continues to be given at 1.8 mg/kg (to a maximum dose of 180 mg) every 3 weeks. Dosing of brentuximab vedotin beyond 1 year (ie, a total of 16 doses) has not been extensively studied in ALCL but extended therapy could be explored-especially in asymptomatic patients, given that the median duration of response of just 13 months suggests relapse occurs shortly after cessation of therapy. Repeat responses have been achieved in patients who have responded to brentuximab vedotin, completed 1 year of therapy, and then relapsed.[30] As previously noted in the discussion of induction therapy for patients with ALCL, the outstanding results seen with brentuximab vedotin have quickly propelled this drug into the upfront treatment setting, and comparative data are eagerly awaited. In contrast to Hodgkin lymphoma regimens that include brentuximab vedotin, cost-benefit analysis of treatment with brentuximab vedotin in the setting of ALCL is not likely to be necessary. Brentuximab vedotin has also been explored as a single agent in other CD30-expressing or nonexpressing subtypes of PTCL. The relationship between percentage of CD30 expression by immunohistochemistry and response to brentuximab vedotin has become less clear. Horwitz et al described several cases of AITL in which CRs were still achieved despite a lack of CD30 expression by immunohistochemistry.[31] Paradoxically, several patients with higher CD30 expression did not derive a benefit. Further investigation into this discrepancy and evaluation of technologies to better assess CD30 expression are ongoing.
The most recent drug to be approved in rel/ref PTCL is the histone deacetylase inhibitor belinostat. In an early phase II study that included patients with cutaneous T-cell lymphoma (n = 14), the ORR was 25% in the cohort with PTCL (n = 25).[32] A follow-up phase II study in 120 patients reported a similar ORR of 25.8%, with 10.8% achieving a CR. The median duration of response was 8.4 months.[33,34] Similar to romidepsin, toxicities included nausea, vomiting, and mild hematologic toxicities. The administration schedule for belinostat is different from that of romidepsin; it is given as an intravenous infusion on days 1 to 5 of a 3-week cycle.
Lastly, for patients with rel/ref PTCL, potential curative options include consolidation with either an ASCT or allogeneic stem cell transplant if this process has not already been completed. Unlike in other B-cell NHLs, an experience with second ASCT has not been reported in PTCL.[35] While allogeneic stem cell transplant is often appropriately considered and offered, few patients undergo this procedure due to the highly refractory nature of PTCL. Early referral to a transplant center is recommended in order to lower the odds of missing a short window of time in which a transplant could be completed. The literature on allogeneic stem cell transplantation in PTCL remains retrospective and relatively heterogeneous. Goldberg and colleagues reported a single-center experience of 34 patients with PTCL; with a median follow-up of nearly 4 years, the 2-year OS was 61%.[36] Interestingly, a plateau at 28 months was observed and, as expected, showed that patients who entered the study with a CR had a significantly improved OS time. Upfront allogeneic stem cell transplant remains an option for exploration in trials including patients with common subtypes of PTCL.
Conclusion
While PTCL remains uncommon in North America, when a diagnosis is rendered by an experienced hematopathologist, the patient can be more confident that the diagnosis is correct. Furthermore, molecular studies may be on the horizon to help better define subtype assignment.[37]
Common subtypes continue to dominate the prospective literature in all phases of care for patients with PTCL. To date, anthracycline-based chemotherapy remains an essential component of care outside of a clinical trial. Several novel agents in combination with anthracycline-based regimens are now in phase III trials, challenging traditional treatment with CHOP. The question that remains to be answered is whether a positive study in favor of a novel agent plus CHOP when compared with CHOP alone will translate to increased use of said “winner” when options such as CHOEP or DA-EPOCH remain available. In regard to clinical trials in this setting, it would be ironic if, in a disease in which four phase II trials led to FDA approval of four agents in less than a decade, the existence of multiple concurrent phase III studies competing for scarce PTCL patients led to study closures and underpowered analysis. Although consideration of consolidative ASCT in first remission remains an attractive option for eligible patients, those who are not exposed to brentuximab vedotin in the first-line setting may elect to defer transplant unless relapse is seen, specifically in ALCL. Outside of brentuximab vedotin in ALCL, in the rel/ref setting we continue to see ORRs of 30%; therefore many patients will quickly proceed through each treatment. If a response is achieved, however, then durable responses can be realized with each of the novel agents. Allogeneic stem cell transplantation is a curative option after relapse or in the refractory setting. Early referral and communication with the transplant physician is paramount to avoid missing a window of therapeutic opportunity. Lastly, enrollment into clinical trials remains the only approach toward making a gray situation more black and white.
Financial Disclosure:Dr. Lunning is a consultant to Bristol-Myers Squibb (BMS), Celgene, Genentech, Gilead, Spectrum, and TG Therapeutics. He receives research funding from BMS, Janssen, Pharmacyclics, Spectrum, and TG Therapeutics.
References:
1. Swerdlow SH, Campo E, Harris NL, et al, editors: WHO classification of tumours of haematopoietic and lymphoid tissues. 4th ed. Vol 2. Lyon, France: International Agency for Research on Cancer (IARC); 2008.
2. Vose J, Armitage J, Weisenburger D. International peripheral T-cell and natural killer/T-cell lymphoma study: pathology findings and clinical outcomes. J Clin Oncol. 2008;26:4124-30.
3. Gallamini A, Stelitano C, Calvi R, et al. Peripheral T-cell lymphoma unspecified (PTCL-U): a new prognostic model from a retrospective multicentric clinical study. Blood. 2004;103:2474-9.
4. Gisselbrecht C, Gaulard P, Lepage E, et al. Prognostic significance of T-cell phenotype in aggressive non-Hodgkin’s lymphomas. Groupe d’Etudes des Lymphomes de l’Adulte (GELA). Blood. 1998;92:76-82.
5. Tsukasaki K, Utsunomiya A, Fukuda H, et al. VCAP-AMP-VECP compared with biweekly CHOP for adult T-cell leukemia-lymphoma: Japan Clinical Oncology Group Study JCOG9801. J Clin Oncol. 2007;25:5458-64.
6. Savage KJ, Chhanabhai M, Gascoyne RD, Connors JM. Characterization of peripheral T-cell lymphomas in a single North American institution by the WHO classification. Ann Oncol. 2004;15:1467-75.
7. Schmitz N, Trumper L, Ziepert M, et al. Treatment and prognosis of mature T-cell and NK-cell lymphoma: an analysis of patients with T-cell lymphoma treated in studies of the German High-Grade Non-Hodgkin Lymphoma Study Group. Blood. 2010;116:3418-25.
8. d’Amore F, Relander T, Lauritzsen GF, et al. Up-front autologous stem-cell transplantation in peripheral T-cell lymphoma: NLG-T-01. J Clin Oncol. 2012;30:3093-9.
9. Wilson WH, Jung SH, Porcu P, et al. A Cancer and Leukemia Group B multi-center study of DA-EPOCH-rituximab in untreated diffuse large B-cell lymphoma with analysis of outcome by molecular subtype. Haematologica. 2012;97:758-65.
10. Kluin-Nelemans HC, van Marwijk Kooy M, Lugtenburg PJ, et al. Intensified alemtuzumab-CHOP therapy for peripheral T-cell lymphoma. Ann Oncol. 2011;22:1595-600.
11. Corradini P, Vitolo U, Rambaldi A, et al. Intensified chemo-immunotherapy with or without stem cell transplantation in newly diagnosed patients with peripheral T-cell lymphoma. Leukemia. 2014;28:1885-91.
12. Binder C, Ziepert M, Pfreundschuh M, et al. CHO(E)P-14 followed by alemtuzumab consolidation in untreated peripheral T cell lymphomas: final analysis of a prospective phase II trial. Ann Hematol. 2013;92:1521-8.
13. Cheson BD. Staging and response assessment in lymphomas: the new Lugano classification. Chin Clin Oncol. 2015;4:5.
14. Mahadevan D, Unger JM, Spier CM, et al. Phase 2 trial of combined cisplatin, etoposide, gemcitabine, and methylprednisolone (PEGS) in peripheral T-cell non-Hodgkin lymphoma: Southwest Oncology Group Study S0350. Cancer. 2013;119:371-9.
15. Advani R, Ansell SM, Lechowicz MJ, et al. A phase II study of cyclophosphamide, etoposide, vincristine, and prednisone (CEOP) alternating with pralatrexate (P) as front line therapy for patients with peripheral T-cell lymphoma (PTCL): preliminary results for the T-cell Consortium trial. Presented at the 55th ASH Annual Meeting and Exposition. New Orleans, LA; December 7-10, 2013. Abstr 3044.
16. Pro B, Advani R, Brice P, et al. Brentuximab vedotin (SGN-35) in patients with relapsed or refractory systemic anaplastic large-cell lymphoma: results of a phase II study. J Clin Oncol. 2012;30:2190-6.
17. Fanale MA, Horwitz SM, Forero-Torres A, et al. Brentuximab vedotin in the front-line treatment of patients with CD30+ peripheral T-cell lymphomas: results of a phase I study. J Clin Oncol. 2014;32:3137-43.
18. Stiff PJ, Unger JM, Cook JR, et al. Autologous transplantation as consolidation for aggressive non-Hodgkin’s lymphoma. N Engl J Med. 2013;369:1681-90.
19. Rodriguez J, Munsell M, Yazji S, et al. Impact of high-dose chemotherapy on peripheral T-cell lymphomas. J Clin Oncol. 2001;19:3766-70.
20. Mercadal S, Briones J, Xicoy B, et al. Intensive chemotherapy (high-dose CHOP/ESHAP regimen) followed by autologous stem-cell transplantation in previously untreated patients with peripheral T-cell lymphoma. Ann Oncol. 2008;19:958-63.
21. Corradini P, Tarella C, Zallio F, et al. Long-term follow-up of patients with peripheral T-cell lymphomas treated up-front with high-dose chemotherapy followed by autologous stem cell transplantation. Leukemia. 2006;20:1533-8.
22. Reimer P, Rudiger T, Geissinger E, et al. Autologous stem-cell transplantation as first-line therapy in peripheral T-cell lymphomas: results of a prospective multicenter study. J Clin Oncol. 2009;27:106-13.
23. Beitinjaneh A, Saliba RM, Medeiros LJ, et al. Comparison of survival in patients with T cell lymphoma after autologous and allogeneic stem cell transplantation as a frontline strategy or in relapsed disease. Biol Blood Marrow Transplant. 2015;21:855-9.
24. Savage KJ, Harris NL, Vose JM, et al. ALK- anaplastic large-cell lymphoma is clinically and immunophenotypically different from both ALK+ ALCL and peripheral T-cell lymphoma, not otherwise specified: report from the International Peripheral T-Cell Lymphoma Project. Blood. 2008;111:5496-504.
25. Smith SM, Burns LJ, van Besien K, et al. Hematopoietic cell transplantation for systemic mature T-cell non-Hodgkin lymphoma. J Clin Oncol. 2013;31:3100-9.
26. Kewalramani T, Zelenetz AD, Teruya-Feldstein J, et al. Autologous transplantation for relapsed or primary refractory peripheral T-cell lymphoma. Br J Haematol. 2006;134:202-7.
27. Damaj G, Gressin R, Bouabdallah K, et al. Results from a prospective, open-label, phase II trial of bendamustine in refractory or relapsed T-cell lymphomas: the BENTLY Trial. J Clin Oncol. 2013;31:104-10.
28. O’Connor OA, Pro B, Pinter-Brown L, et al. Pralatrexate in patients with relapsed or refractory peripheral T-cell lymphoma: results from the pivotal PROPEL study. J Clin Oncol. 2011;29:1182-9.
29. Coiffier B, Pro B, Prince HM, et al. Results from a pivotal, open-label, phase II study of romidepsin in relapsed or refractory peripheral T-cell lymphoma after prior systemic therapy. J Clin Oncol. 2012;30:631-6.
30. Bartlett NL, Chen R, Fanale MA, et al. Retreatment with brentuximab vedotin in patients with CD30-positive hematologic malignancies. J Hematol Oncol. 2014;7:24.
31. Horwitz SM, Advani RH, Bartlett NL, et al. Objective responses in relapsed T-cell lymphomas with single-agent brentuximab vedotin. Blood. 2014;123:3095-100.
32. Foss F, Advani R, Duvic M, et al. A phase II trial of belinostat (PXD101) in patients with relapsed or refractory peripheral or cutaneous T-cell lymphoma. Br J Haematol. 2015;168:811-9.
33. Lee HZ, Kwitkowski VE, Del Valle PL, et al. FDA approval: belinostat for the treatment of patients with relapsed or refractory peripheral T-cell lymphoma. Clin Cancer Res. 2015 Mar 23. [Epub ahead of print]
34. O’Connor OA, Masszi T, Savage KJ, et al. Belinostat, a novel pan-histone deacetylase inhibitor (HDACi), in relapsed or refractory peripheral T-cell lymphoma (R/R PTCL): results from the BELIEF trial. J Clin Oncol. 2013;31(suppl):abstr 8507.
35. Smith SM, van Besien K, Carreras J, et al. Second autologous stem cell transplantation for relapsed lymphoma after a prior autologous transplant. Biol Blood Marrow Transplant. 2008;14:904-12.
36. Goldberg JD, Chou JF, Horwitz S, et al. Long-term survival in patients with peripheral T-cell non-Hodgkin lymphomas after allogeneic hematopoietic stem cell transplant. Leuk Lymphoma. 2012;53:1124-9.
37. Iqbal J, Wright G, Wang C, et al. Gene expression signatures delineate biological and prognostic subgroups in peripheral T-cell lymphoma. Blood. 2014;123:2915-23.