Multiple Myeloma With Extramedullary Disease: A Challenging Clinical Dilemma
A 39-year-old woman with no significant medical history presented to the emergency department with progressive diffuse abdominal pain, involuntary weight loss, anemic syndrome, and limitation of mobility. What is the best treatment course to follow?
Oncology (Williston Park). 33(4):149-51, 155.

Katherinee Morales-Chacón, MD

María T. Bourlon, MD, MSc

Deborah Martínez-Baños, MD, PhD

Jesús Delgado-de-la-Mora, MD

Christianne Bourlon, MD, MHSc

Figure 1. Contrast CT Scan Imaging of the Abdomen and Pelvis
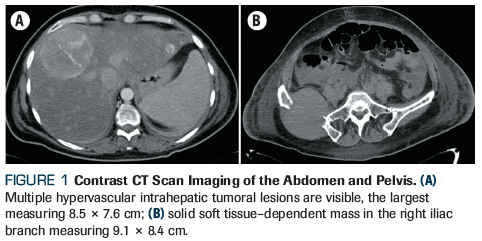
Figure 2. Histopathologic Evaluation of Bone Marrow Biopsy
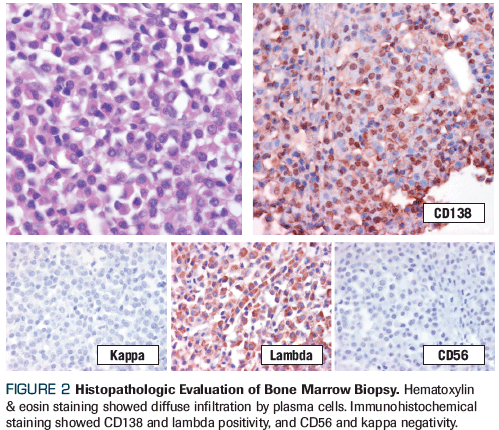
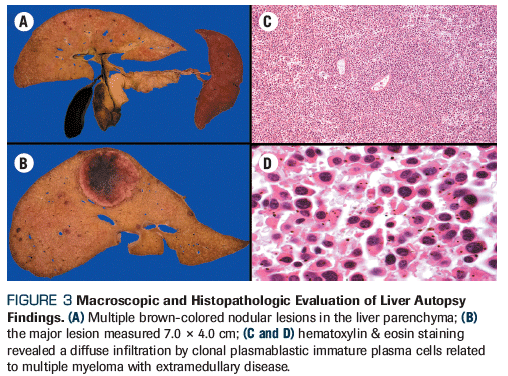
Figure 3. Macroscopic and Histopathologic Evaluation of Liver Autopsy Findings
The Case
A 39-year-old woman with no significant medical history presented to the emergency department with progressive diffuse abdominal pain, involuntary weight loss, anemic syndrome, and limitation of mobility related to the presence of a right gluteal mass, all of which had persisted for the past 20 days. Physical examination revealed hepatomegaly, splenomegaly, ascites, right leg edema, and a palpable 6.0 × 5.3–cm mass in the right gluteus.
Blood tests were obtained following admission, revealing anemia (hemoglobin level, 9.9 g/dL), hyperglobulinemia (globulins level, 11.0 g/dL), hypercalcemia (calcium level, 14.15 mg/dL), and renal failure (creatinine level, 1.4 mg/dL), as well as coagulopathy with prolonged prothrombin time (PT), activated partial thromboplastin time (aPTT), and hypofibrinogenemia (fibrinogen level, 92.0 mg/dL). A CT scan showed ascites; splenomegaly; hepatomegaly dependent on multiple hypervascular intrahepatic tumoral lesions, the largest measuring 8.5 × 7.6 cm (segment 8 of Figure 1A); a solid soft tissue–dependent mass in the right iliac branch measuring 9.1 × 8.4 cm (Figure 1B); and multiple skeletal lytic lesions.
Due to clinical suspicion of monoclonal gammopathy, the following tests were performed: serum quantitative immunoglobulin (Ig) levels (IgA level, 14.0 mg/dL; IgM level, 20.0 mg/dL; IgG level, 6,842 mg/dL); beta-2 microglobulin level (10.5 mg/dL); serum free light chains (kappa free light chains, 10.0 mg/L; lambda free light chains, 7,500 mg/L; kappa/lambda ratio, 0.002); and serum protein electrophoresis and immunofixation, which showed an IgG lambda monoclonal paraprotein (6.8 g/dL).
Bone marrow trephine biopsy established the presence of 60% lambda clonal plasma cells (CD138-positive, CD56-negative, cyclin D1–negative; Figure 2), confirming a diagnosis of IgG lambda multiple myeloma. On cytogenetic analysis, the presence of chromosome 9 inversion and chromosome 17p11.2 deletion were identified: karyotype 5 = 46,XX,inv(9)(p13q13), del(17)(p11.2p11.2); karyotype 15 = 46,XX,inv(9)(p13q13). Fluorescence in situ hybridization from the bone marrow ruled out additional cytogenetic abnormalities. Peripheral blood immunophenotype ruled out plasma cell leukemia (4.8% CD138-positive plasma cells).
Regarding the presence of coagulopathy, additional tests were performed; the results of a thrombin time test were normal, and a mixing study found a correction of both PT and aPTT. Therefore, quantification of factor II (FII), factor V (FV), and factor X (FX) levels was completed, all of which were low (FII, 26%; FV, 50.7%; FX, 27.5%). During the visit, the patient demonstrated mild mucocutaneous bleeding and was treated with fresh frozen plasma transfusion and cryoprecipitate without clinical instability.
Within days of the initial visit, the patient presented with clinical deterioration, including worsened renal failure requiring hemodialysis, coagulopathy, and disseminated candidiasis that led to death. After the patient passed away, a scientific medical autopsy was performed. Macroscopic findings complemented with histopathology tests were consistent with multiple myeloma with extramedullary disease (EMD) affecting the liver (Figure 3), gluteus muscle, ovaries, kidneys, and peritoneum.
Which of the following hematopoietic stem cell transplant (HSCT) approaches would have been the best choice after initial chemotherapy in this case?
A. Single autologous HSCT
B. Tandem autologous HSCT
C. Allogeneic HSCT
D. Tandem autologous HSCT followed by allogeneic HSCT
Correct Answer: A. Single autologous HSCT
Discussion
Multiple myeloma is a hematologic neoplasia characterized by a proliferation of clonal plasma cells, as well as evidence of related end organ damage. In most cases, the rapid reproduction of clonal plasma cells is restricted to the bone marrow; however, in some cases they can spread outside the bone marrow in patients diagnosed with multiple myeloma, which is recognized as EMD.[1-3] Currently, many questions remain regarding the clinical behavior, prognosis, and treatment of patients with EMD.
Over the last 2 decades, continuous debate has surrounded the definition of EMD. Initially, two clinical presentations were referred to as EMD: 1) a tumor mass adjacent to the bone that extends to the soft tissue and bone marrow (bone-related plasmacytoma), and 2) a tumor mass or diffuse infiltration by clonal plasma cells at a distant anatomical site not related to the bone.[4,5] In 2013, Weinstock and Ghobrial proposed that, taking into consideration the biological behavior and prognostic characteristics of this disease presentation, the term EMD should be restricted to the presence of EMD in patients with multiple myeloma, excluding bone-related plasmacytoma.[6]
The reported rate of patients presenting with EMD varies between 6% and 30%, depending on the time of EMD diagnosis, sites considered to be EMD, and the diagnostic method used for identification. When using adequate imaging techniques (ie, whole-body MRI or PET/CT), the incidence of EMD is approximately 7%.[2,5,7] In the relapsed/refractory setting, the prevalence of EMD has been reported to be as high as 10% to 30%; however, its real prevalence could be much higher when considering that autopsy studies have revealed extramedullary involvement in approximately 70% of suspected cases, with up to 40% infiltration to non–bone-related distant sites.[2,8-10]
To our knowledge, multiple myeloma with EMD affecting multiple distant-to-the-bone sites at diagnosis is rare; hepatic and ovarian involvement are particularly rare, with only a few cases reported in the literature.[11-13] In 2018, the European Society for Blood and Marrow Transplantation (EBMT) conducted a retrospective study that included 3,744 patients with multiple myeloma. Bone-related EMD was identified in 14.5% of the patients, only 3.7% of whom had non-paraskeletal involvement. The frequency of involved extramedullary sites was as follows: kidney (27.3%); skin (23%); lymph nodes (17.3%); central nervous system (10.1%); lung and respiratory tract (6.5%); gastrointestinal tract and liver (5.8%); pleura and heart (5.0%); and spleen, ovaries, and testicles (5.3%). In addition, 93.5% of patients presented with one involved site and only 6.5% with two or more.[7,14]
At the histopathology level, clonal plasma cells have an immature/plasmablastic appearance in patients with EMD, while they have a mature/plasmacytic appearance in bone-related plasmacytoma. Differences in morphology, as well as the deleterious clinical impact of EMD, suggest that the pathogenesis of these two presentations is different. Although the exact mechanisms that contribute to the spread of clonal plasma cells is unknown, some pathophysiologic mechanisms have been proposed. Oncogene and tumor suppressor gene mutations that affect protein expression related to cell survival, migration, and invasion play a crucial role in patients with EMD. Clonal mutations, mainly of the TP53, RB1, FAK, and RAS genes, have been described in up to 50% of patients with multiple myeloma with an EMD presentation.[2,4,6,15]
In EMD, migration of plasma cells to distant sites has also been related to the upregulation or downregulation of different molecules. Regarding adhesion molecules, CD56 and P-selectin promoters of the adherence of cells to the bone marrow are markedly unexpressed, while CD44-involved in proliferation, migration, and tumor metastasis-was found to have increased expression. Angiogenesis- and hypoxia-related factors (VEGF, MMP-9, angiopoietin-1, and CD31) and dysregulation of cytosines (CCR1, CCR2, and CXCR-4/SDF-1α) are still undergoing laboratory and clinical research, but have been described as specific pathogenic steps that limit or promote hematogenous dissemination of plasma cells.[15,16]
Multiple myeloma with EMD is known to have a highly aggressive disease presentation at both the time of diagnosis and relapse.[5,17] Usmani et al reported that EMD at diagnosis was associated with a significantly lower progression-free survival rate at 5 years compared with multiple myeloma without EMD, regardless of treatment modality (21% vs 50%; P < .001).[18] More recently, the EBMT evaluated the impact of EMD on newly diagnosed multiple myeloma patients undergoing autologous hematopoietic stem cell transplantation (auto-HSCT). They found that patients with EMD had significantly lower rates of progression-free survival (39.9% vs 47.9%; P = .001) and overall survival (58% vs 80.1%; P < .001) at 3 years compared with patients without EMD. They also observed that the progression-free survival rate at 3 years was similar among patients with EMD with involvement of a unique site and patients without EMD (49.4% vs 47.9%, respectively; P = .36). However, when compared with cases involving multiple disease sites, patients with EMD had worse outcomes (47.9% vs 22.7%, respectively; P = .001).[14]
Considering the adverse impact that EMD has on clinical outcomes, the molecular and cytogenetic heterogeneity of multiple myeloma, and the lack of EMD-specific predictive scores, it is imperative that diagnostic strategies for this disease presentation be optimized. According to the current international recommendations, radiographs and MRI are acceptable methods for initial screening[19,20]; however, PET/CT has been recognized as a more effective technique, not only at diagnosis, but also during patient follow-up. Recent studies have demonstrated that PET/CT is a more sensible and specific tool to assess bone damage and extramedullary sites; distinguish between metabolically active and inactive disease; and determine response to therapy, including detection of minimal residual disease.[21-23]
Coagulopathy presenting in parallel with multiple myeloma has been described in 15% to 40% of cases.[24-26] Major hemorrhagic complications are rare, with clinically significant bleeding occurring in less than 15% of patients, with no relation to the degree of laboratory test abnormalities. Risk factors related to bleeding include increased serum viscosity and levels of monoclonal paraprotein, as well as conditions that interfere with platelet function, coagulation factors, and fibrinolysis.[27,28] Incidence of coagulopathy in patients with EMD is unknown; nevertheless, previous reports have linked hemostatic disorders with high-risk multiple myeloma.[29,30] Supportive care and timely control of primary neoplasia have been the only effective strategies to control related symptoms and complications.[24,31]
One limitation of current therapy recommendations is that no prospective clinical trials have focused specifically on multiple myeloma patients with EMD. Expert opinion favors considering EMD to be a high-risk disease feature, and therefore to treat it with an intensive regimen. In this context, the preferred regimen for a newly diagnosed transplant candidate is triplet induction therapy including a proteasome inhibitor and an immunomodulator (carfilzomib or bortezomib with lenalidomide/dexamethasone), followed by auto-HSCT with high-dose melphalan conditioning, triplet consolidation therapy, and maintenance therapy with lenalidomide (Answer A).[3] For non-transplant candidates, standard-of-care triplet therapy with an alkylating agent and proteasome inhibitor (bortezomib plus melphalan and prednisone), or continuous lenalidomide with dexamethasone, are the most effective therapies with high response rates.[32]
Routine use of tandem auto-HSCT (Answers B and D) has been proposed by some groups. Evidence in patients with EMD is scarce, since the evidence supporting its use was based on its utility in high-risk patients.[33] Allogeneic HSCT (Answer C) has been reserved for patients with disease that does not respond to therapy or those who experience relapse after initial therapy with auto-HSCT.[33,34] Although there are no prospective data, current ongoing trials evaluating various upfront therapies have been integrating the use of PET/CT into the diagnosis and follow-up of patients-a strategy that will hopefully aid in more precisely analyzing response to therapy and clinical outcomes in patients with EMD.
Financial Disclosure:Dr. Maria Bourlon serves on the advisory boards and speakers’ bureaus of and receives travel grants from Bristol-Myers Squibb and Janssen. All other authors have no significant financial interest in or other relationship with the manufacturer of any product or provider of any service mentioned in this article.
References:
1. Rajkumar SV, Dimopoulos MA, Palumbo A, et al. International Myeloma Working Group updated criteria for the diagnosis of multiple myeloma. Lancet Oncol. 2014;15:e538-48.
2. Bladé J, Fernández de Larrea C, Rosiñol L, et al. Soft-tissue plasmacytomas in multiple myeloma: incidence, mechanisms of extramedullary spread, and treatment approach. J Clin Oncol. 2011;29: 3805-12.
3. Touzeau C, Moreau P. How I treat extramedullary myeloma. Blood. 2016;127:971-6.
4. Short KD, Rajkumar SV, Larson D, et al. Incidence of extramedullary disease in patients with multiple myeloma in the era of novel therapy, and the activity of pomalidomide on extramedullary myeloma. Leukemia. 2011;25:906-8.
5. Varettoni M, Corso A, Pica G, et al. Incidence, presenting features and outcome of extramedullary disease in multiple myeloma: a longitudinal study on 1003 consecutive patients. Ann Oncol. 2010;21:325-30.
6. Weinstock M, Ghobrial IM. Extramedullary multiple myeloma. Leuk Lymphoma. 2013;54:1135-41.
7. Weinstock M, Aljawai Y, Morgan EA, et al. Incidence and clinical features of extramedullary multiple myeloma in patients who underwent stem cell transplantation. Br J Hematol. 2015;169:851-8.
8. Varga C, Xie W, Laubach J, et al. Development of extramedullary myeloma in the era of novel agents: no evidence of increased risk with lenalidomide-bortezomib combinations. Br J Hematol. 2015;169:843-50.
9. Pasmantier MW, Azar HA. Extraskeletal spread in multiple plasma cell myeloma. A review of 57 autopsied cases. Cancer. 1969;1:167-74.
10. Thomas FB, Clausen KP, Greenberger NJ. Liver disease in multiple myeloma. Arch Intern Med. 1973;132:195-202.
11. Huang H, Bazerbachi F, Mesa H, and Gupta P. Asymptomatic multiple myeloma presenting as a nodular hepatic lesion: a case report and review of the literature. Ochsner J. 2015;15:457-67.
12. Chemlal K, Couvelard A, Grange MJ, et al. Nodular lesions of the liver revealing multiple myeloma. Leuk Lymphoma. 1999;33:389-92.
13. Stuver R, Petersen A, Guerrero-Garcia TA, et al. Multiple myeloma masquerading as ovarian carninosarcoma metastases: a case report and review of the approach to multiple myeloma screening and diagnosis. Case Rep Hematol. 2018 Sep 24. [Epub ahead of print]
14. Gagelmann N, Eikema DJ, Iacobelli S, et al. Impact of extramedullary disease in patients with newly diagnosed multiple myeloma undergoing autologous stem cell transplantation: a study from the Chronic Malignancies Working Party of the EBMT. Haematologica. 2019;103:890-7.
15. Rasche L, Bernard C, Topp M, et al. Features of extramedullary myeloma relapse: high proliferation, minimal marrow involvement, adverse cytogenetics: a retrospective single-center study of 24 cases. Ann Hematol. 2012;91:1031-7.
16. Bink K, Haralambieva E, Kremer M, et al. Primary extramedullary plasmacytoma: similarities and differences with multiple myeloma revealed by interphase genetics. Haematologica. 2008;93:623-6.
17. Rosiñol L, Oriol A, Teruel AI, et al. Superiority of bortezomib, thalidomide, and dexamethasone (VTD) as induction pretransplantation therapy in multiple myeloma: a randomized phase 3 PETHEMA/GEM study. Blood. 2012;120:1589-96.
18. Usmani SZ, Rodriguez-Otero P, Bhutani M, et al. Defining and treating high-risk multiple myeloma. Leukemia. 2015;11:2119-25.
19. Walker RC, Brown TL, Jones-Jackson LB, et al. Imaging of multiple myeloma and related plasma cell dyscrasias. J Nucl Med. 2012;53:1091-101.
20. Dimopoulos MA, Hilengass J, Usmani S, et al. Role of magnetic resonance imaging in the management of patients with multiple myeloma: a consensus statement. J Clin Oncol. 2015;33:657-64.
21. Bartel TB, Haessler J, Brown TL, et al. F-18 fluorodeoxyglucose positron emission tomography in the context of other imaging techniques and prognostic factors in multiple myeloma. Blood. 2009;114:2068-76.
22. Cavo M, Terpos E, Nanni C, et al. Role of 18F-FDG PET/CT in the diagnosis and management of multiple myeloma and other plasma cell disorders: a consensus statement by the International Myeloma Working Group. Lancet Oncol. 2017;18:e206-17.
23. Nanni C, Zamagni E, Versari A, et al. Image interpretation criteria for FDG PET/CT in multiple myeloma: a new proposal from an Italian expert panel. IMPeTUs (Italian Myeloma criteria for PET USe). Eur J Nucl Med Mol Imaging. 2016;43:414-21.
24. Coppola A, Tufano A, Di Capua M, Franchini M. Bleeding and thrombosis in multiple myeloma and related plasma cell disorders. Semin Thromb Hemost. 2011;37:929-45.
25. Perkins HA, MacKenzie MR, Fudenberg HH. Hemostatic defects in dysproteinemias. Blood. 1970;35:695-707.
26. Mehta J, Singhal S. Hyperviscosity syndrome in plasma cell dyscrasias. Semin Thromb Hemost. 2003;29:467-71.
27. Shinagawa A, Kojima H, Berndt MC, et al. Characterization of a myeloma patient with a life-threatening hemorrhagic diathesis: presence of a lambda dimer protein inhibiting shear-induced platelet aggregation by binding to the A1 domain of von Willebrand factor. Thromb Haemost. 2005;93:889-96.
28. Teng HW, Chen PM, Yang YH, Gau JP. The prolonged activated partial thromboplastin time at diagnosis indicates less favorable prognosis in IgA myeloma. Jpn J Clin Oncol. 2007;37:609-14.
29. van Marion AM, Auwerda JJ, Lisman T, et al. Prospective evaluation of coagulopathy in multiple myeloma patients before, during and after various chemotherapeutic regimens. Leuk Res. 2008;32:1078-84.
30. Glaspy JA. Hemostatic abnormalities in multiple myeloma and related disorders. Hematol Oncol Clin North Am. 1992;6:1301-14.
31. Sonneveld P, Avet-Loiseau H, Lonial S, et al. Treatment of multiple myeloma with high-risk cytogenetics: a consensus of the International Myeloma Working Group. Blood. 2016;127:2955-62.
32. Mateus MV, Oriol A, MartÃnez-López J, et al. Outcomes with two different schedules of bortezomib, melphalan, and prednisone (VMP) for previously untreated multiple myeloma: matched pair analysis using long-term follow-up data from the phase 3 VISTA and PETHEMA/GEM05 trials. Ann Hematol. 2016;95:2033-41.
33. Cavo M, Salwender H, Rosiñol L, et al. Double vs single autologous stem cell transplantation after bortezomib-based induction regimens for multiple myeloma: an integrated analysis of patient-level data from phase European III studies. Blood. 2013;122:767.
34. Badros A, Barlogie B, Siegel E, et al. Results of autologous stem cell transplant in multiple myeloma patients with renal failure. Br J Haematol. 2001;114:822-9.

Navigating AE Management for Cellular Therapy Across Hematologic Cancers
A panel of clinical pharmacists discussed strategies for mitigating toxicities across different multiple myeloma, lymphoma, and leukemia populations.