Novel Targets and Precision Medicine for Prostate Cancer-Part 2: Tumor Profiling and Personalized Therapy in Patients With Castration-Resistant Prostate Cancer
In this article, we review the current use of and future direction for genetic testing and tumor profiling in patients with metastatic castration-resistant prostate cancer.
Oncology (Williston Park). 33(4):128-31.

Bryden Considine, DO

Daniel P. Petrylak, MD

Table 1. Ready-for-Practice Therapeutic Agents for the Second-Line Treatment of CRPC
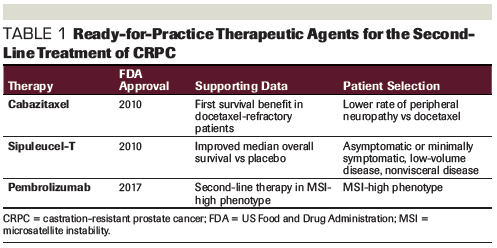
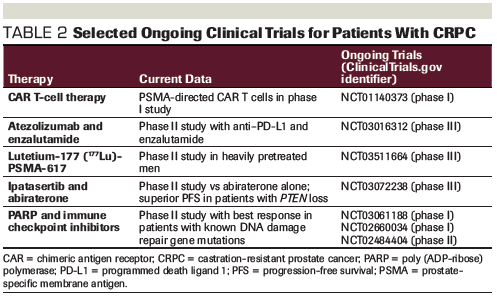
Table 2. Selected Ongoing Clinical Trials for Patients With CRPC
Despite advances in the treatment of castration-resistant prostate cancer (CRPC), options remain limited and non-curative; thus, prostate cancer remains one of the deadliest cancers in men. The discovery of novel therapeutic targets is needed to improve outcomes for men with metastatic CRPC. Precision/personalized medicine creates new opportunities to discover these targets. With an increase in the use of next-generation sequencing and tumor profiling, potentially clinically relevant tumor mutations are being identified. Here, we review the current use of and future direction for genetic testing and tumor profiling in patients with metastatic CRPC.
Introduction
Despite the success of androgen blockade, nearly all metastatic prostate cancer patients progress to castration-resistant disease. Although treatment of castration-resistant prostate cancer (CRPC) has improved substantially, most patients succumb to their disease. In Part 1 of this two-part review, we reviewed novel targeted therapies and immuno-oncology agents, as well as the future role of biomarker-driven studies in patients with CRPC (visit CancerNetwork.com/CRPC-part-1). As DNA sequencing techniques improve and become more available in the clinic, we have identified common mutations with predictive implications for the management of these patients. In Part 2, we review the current use of genetic and tumor profiling and its role in providing more personalized treatment strategies.
Microsatellite Instability
Pembrolizumab is the only immune checkpoint inhibitor that has been approved by the US Food and Drug Administration (FDA) across the spectrum of solid tumors, based on the presence of microsatellite instability (MSI). Defects in DNA mismatch repair (MMR) genes lead to an MSI-high phenotype that is associated with an increased mutational burden. Despite overall poor responses to anti–programmed death 1 (PD-1) therapy in colon cancer, a complete response was observed in a patient with metastatic colon adenocarcinoma and an MSI-high phenotype.[1] This finding led to a phase II study with pembrolizumab that demonstrated improved survival in mismatch repair–deficient colorectal cancer patients. This is thought to be secondary to an increased number of mutation-associated neoantigens seen in the mismatch repair–deficient population.[2] Subsequently, the efficacy of pembrolizumab in the MSI-high phenotype was investigated in five uncontrolled, open-label, single-arm trials, which led to its FDA approval in this setting. Of note, there were 2 patients with metastatic prostate cancer, 1 with stable disease, and 1 with a partial response.[3]
The correlation between MMR mutations and responses to immune checkpoint inhibition raises important questions for patients with prostate cancer. Pritchard et al demonstrated that 12% of patients with advanced prostate cancer have a hypermutated subtype, and that hypermutated cancers are associated with MMR mutations (MSH2 or MSH6).[4] In another study, the frequency of MSI in primary prostate cancer was investigated by immunohistochemistry and next-generation sequencing of MSH2; MSH2 loss was seen in 8% of Gleason pattern 5 (Gleason score, 9–10) primary adenocarcinomas of the prostate compared with 0.4% in all other Gleason scores. In addition, patients with MSH2 loss were found to have an increase in CD8+ lymphocyte density, which may predict a response to immune checkpoint inhibition.
The actual prevalence of all MMR mutations in patients with Gleason score 9–10 is likely even higher than that seen with MSH2 mutations alone and supports the idea that screening for MSI should be performed in this patient population.[5] Early results from a study that included patients with either MSI or programmed death ligand 1 (PD-L1) positivity (> 1%) treated with either nivolumab or pembrolizumab have shown a biochemical prostate-specific antigen (PSA) response (> 50%).[6] An ongoing prospective clinical trial (ClinicalTrials.gov identifier: NCT02966587) will evaluate how patients with MSI respond to immune checkpoint inhibition with durvalumab. The National Comprehensive Cancer Network guidelines now recommend treatment with pembrolizumab for patients with metastatic prostate cancer with MSI who have progressed on prior systemic therapy.[7] Current FDA-approved second-line treatments for CRPC are summarized in Table 1.
DNA Repair
DNA repair is essential in maintaining the integrity of the genome. Defects in DNA repair promote carcinogenesis and have been identified in at least 20% to 25% of patients with metastatic CRPC.[8] Mutations in germline DNA repair genes have been associated with an increased risk of familial prostate cancer. These genes include BRCA1, BRCA2, MLH1, MSH2, PMS2, NBS1, BRIP1, PALB2, MUTYH, ATM, and CHEK2. [9]
In a study by Pritchard et al of 692 men with metastatic prostate cancer, DNA repair gene mutations were identified in 11.8%. This is significantly higher than the 4.6% prevalence seen among men with localized prostate cancer, which supports a link between mutations in DNA repair genes and a metastatic phenotype. Among these mutations, the most frequently identified was BRCA2 in 5.3% of men.[10] There is also evidence that the presence of BRCA1 or BRCA2 is associated with a high Gleason score (≥ 8), higher incidence of metastatic disease at diagnosis, and a decrease in cause-specific survival. The study by Castro et al found that cause-specific survival was 8.6 years among 61 patients with BRCA2 mutations and 18 patients with BRCA1 mutations compared with 15.7 years in patients without these mutations.[11] In a study of 67 patients definitively treated with either radical prostatectomy or external beam radiation, BRCA1/2 mutations were identified as an independent risk factor for metastasis-free survival, with a hazard ratio of 2.36 compared with non-carriers.[12] These data support that BRCA is associated with an increased risk of metastasis and decreased survival in patients with prostate cancer.
When faced with a single-strand break in DNA, pathways exist to repair the error using the contralateral DNA strand. Repairing a double-stranded DNA break is achieved by the high-fidelity homologous recombination mechanism or the more error-prone nonhomologous end-joining pathway. If DNA repair is unsuccessful, cells enter programmed cell death.[13] Cells that are lacking DNA repair mechanisms will continue DNA replication without error correction, leading to increased carcinogenesis. It is by this mechanism that defects in DNA repair genes lead to an increased risk of cancer.
One of the proteins responsible for initiating repair of single-stranded DNA breaks is poly (ADP-ribose) polymerase (PARP). The PARP protein locates the DNA defect and binds at the replication fork until DNA repair is started.[14] PARP has been an attractive pharmaceutical target, and the first PARP inhibitor developed was olaparib. It was observed that PARP inhibition led to cellular toxicity in cell models deficient of BRCA1/2; this was not observed in cells with heterozygous or wild-type BRCA1/2.[15] It is hypothesized that single-stranded DNA breaks will progress to double-stranded breaks with PARP inhibition. In a system with BRCA-deficient DNA repair mechanisms, these double-stranded DNA breaks cannot be repaired and lead the cell into programmed cell death.[16] In patients with germline BRCA mutations, only the tumor itself is homozygous for BRCA. This allows for PARP-directed therapy against abnormal cancer cells that completely lack BRCA, without affecting normal host cells in patients with BRCA mutations.[17]
The first clinical trial of olaparib included 3 patients with CRPC, with 1 patient with a BRCA2 mutation who remained on the therapy with clinical benefit for over 3 years.[17] A phase II trial of olaparib in patients with metastatic CRPC included 50 patients who had previously progressed on standard therapy. All patients had received docetaxel, 49 had received either abiraterone or enzalutamide, and 29 had received cabazitaxel. Of these patients, 16 (33%) had a response that met RECIST criteria, and 12 patients remained on the study for longer than 6 months.Next-generation sequencing was performed in 49 patients; defects in DNA repair genes, including BRCA1/2, ATM, Fanconi anemia genes, and CHEK2, were identified in 16 of these patients. Among these 16 patients, 14 had a response to olaparib, including all 7 of the patients with loss of BRCA2, and 4 of the 5 with an ATM mutation. Radiographic progression–free survival was 9.8 months in patients with DNA repair gene defects vs 2.7 months in patients without, and overall survival was 13.8 months vs 7.5 months, respectively.[18] These data support the clinical benefit of PARP inhibitors in patients with DNA repair gene mutations who have progressed on standard treatment for metastatic CRPC.
A potentially important treatment option that is currently being investigated is the concurrent use of platinum-based chemotherapy and PARP inhibition. DNA damage caused by the platinum in combination with PARP inhibition of DNA repair should lead to increased cell death. Olaparib in combination with cisplatin has had promising antitumor activity in patients with germline BRCA1/2 mutations in breast and ovarian cancer; however, this combination was associated with significant hematologic toxicities, including neutropenia and anemia, limiting its clinical use.[19] Thus far, studies in patients with prostate cancer have not shown clinical benefit. Further investigation is warranted in this area.
The role of PARP in DNA damage repair is well established; however, it has also been directly associated with a protumorigenic effect via promoting androgen receptor (AR) transcriptional function in AR-positive prostate cells.[20] PARP also regulates prostate cancer cell growth through ETS gene fusion product ERG, since ERG-positive xenografts are sensitive to PARP inhibitors.[21-22] Asim et al demonstrated the role of the AR in maintaining homologous DNA repair gene activity in prostate cancer. With androgen blockade, PARP is upregulated in prostate cancer cells, and high PARP activity is seen in patients treated with androgen deprivation therapy. Synthetic lethality was observed with olaparib in combination with the anti-androgens bicalutamide and enzalutamide.[23] These findings have led to studies that are investigating whether a combination of PARP inhibitors and AR blockade have a role in the treatment of prostate cancer. In one study, 148 patients with metastatic prostate cancer were randomized to abiraterone alone or abiraterone and veliparib. Although a trend was identified favoring the combination, this was not found to have statistical significance.[24] In a randomized phase II study, all patients received abiraterone combined with either olaparib 300 mg or placebo. The median progression-free survival was 13.8 months with olaparib and abiraterone compared with 8.2 months with abiraterone alone.[25]
The combination of PARP and immune checkpoint inhibitors is an area of growing research. In PARP-sensitive tumor cells, DNA damage leads to cell death and antigen release. Of the tumor cells that may survive, it is hypothesized that DNA damage may increase the number of neoantigens, and this combination may lead to an increased availability for recognition by T cells.[26,27] In a phase II trial with durvalumab and olaparib, 8 of 17 prostate cancer patients experienced a reduction in PSA level > 50%. Of these 8 patients, 6 patients had known mutations in DNA damage repair genes.[28] This combination is being investigated in multiple prospective clinical trials (ClinicalTrials.gov identifiers: NCT02484404, NCT03061188, NCT02660034).
Next-Generation Sequencing
Precision medicine powered by next-generation sequencing is a growing area of oncology as demonstrated by the focus on targeted and personalized cancer therapies. By identifying actionable mutations within a tumor, a treatment plan can be developed that targets this underlying driver mutation. This strategy has had success in non–small-cell lung cancer, with EGFR, ALK, ROS1, and BRAF as actionable targets, and has led to the increased use of tumor sequencing in this population. Currently, the molecular landscape of metastatic prostate cancer has had little influence on treatment strategies.
Recent large-scale studies that sequence tumors from patients with CRPC have identified mutations in the AR that lead to resistance to anti-androgen therapies, as well as an increased prevalence of DNA damage repair gene mutations.[29] Twenty percent of patients with CRPC have been shown to have alterations in DNA damage repair genes, including BRCA1, BRCA2, and ATM; patients with BRCA2 mutations have a worse prognosis than noncarriers.[30-34] Recent success with the PARP inhibitor olaparib in patients with metastatic CRPC and DNA damage repair gene mutations has highlighted the importance of next-generation sequencing.
In light of these advances within precision medicine, the question now becomes how to best integrate these tests into clinical practice. While the cost of genetic testing and tumor profiling limits its availability upfront, the clinical benefit seen in many of these early trials is promising. In addition to patients with refractory disease, there are other patient populations that may benefit from upfront testing. In patients with a significant family history that supports a BRCA phenotype, genetic testing can identify these DNA repair mutations, which are associated with an unfavorable prognosis, and this will help identify patients who may benefit from PARP inhibition with olaparib. In addition to the discovery of DNA damage repair mutations, mutations in the AR, which lead to anti-androgen resistance, are being identified and may have therapeutic implications in the near future. Unlike with non–small-cell lung cancer, the molecular landscape of metastatic CRPC has had a limited effect on clinical outcomes for most men and remains a continued focus of further research.
Conclusion
Despite significant advances in the treatment of CRPC, most patients succumb to their disease. In the era of precision medicine and personalized cancer therapy, we are discovering genetic drivers of prostate cancer and uncovering novel therapeutic targets. More clinical trials have incorporated next-generation sequencing and tumor profiling (Table 2). Further characterization of the molecular landscape of prostate cancer has the promise to prolong survival and limit unnecessary treatments through a more personalized cancer treatment plan.
Financial Disclosure:Dr. Petrylak receives consultant fees and grant support from Ada Cap, Astellas, AstraZeneca, Bayer, Bristol-Myers Squibb, Clovis, Eli Lilly, Pfizer, Roche Laboratories, and Seattle Genetics; consultant fees from Amgen, Boehringer Ingelheim, Exelixis, Incyte, Janssen, Pharmacyclics, and Urogen; grant support from Endocyte, Genentech, Innocrin, MedImmune, Merck, Novartis, Progenics, and Sanofi Aventis; and has ownership interest in Bellicum and Tyme. Dr. Considine has no significant financial interest in or other relationship with the manufacturer of any product or provider of any service mentioned in this article.
PERSPECTIVE

Following Genetic Clues: The Future Is Here
A. Karim Kader, MD, PhD, FRCSC
Cancer is a genetic disease. Carcinogenesis, response to therapy, and overall outcome are dependent on the complex interplay between the host and its environment. Many clues regarding these interactions exist within germline and somatic DNA. With the burgeoning research in prostate cancer, together with improved and lower-cost sequencing technologies, reading these genetic tea leaves is now becoming a reality. Considine and Petrylak do a wonderful job outlining the current and future state of genetic testing used to make treatment decisions for the second-line systemic treatment of men with advanced prostate cancer.
Practical application of these technologies can, however, be challenging. There are an increasing number of commercially available tests with variable coverage of known germline mutations. A well-validated companion diagnostic test is necessary for the ultimate success of these targeted therapies. In addition, due to the implications for the patient and his biologic relatives regarding risk of other malignancies, an informed conversation should be had with the patient prior to testing (preferably with a genetic counselor). Furthermore, the polyclonality of these cancers, together with variable sites of disease, make access to somatic DNA difficult. In a perfect world, a well-validated “liquid biopsy” test, representative of the somatic DNA changes related to treatment response, would be available.
In short, the future is here! We are now able to rationally target cancerous mutations with currently available medicines, resulting in improved patient outcomes. However, this excitement needs to be tempered by a clear understanding of and communication about the limitations of this technology. Finally, outside of clinical trials, clinicians should offer this form of testing to only patients in whom actionable information may be gained.
Financial Disclosure: Dr. Kader is the Chief Medical Officer of Stratify Genomics.
Dr. Kader is a Professor of Urology and the Director of Urologic Oncology at Moores Cancer Center, University of California San Diego Health System, San Diego, California.
References:
1. Lipson EJ, Sharfman WH, Drake CG, et al. Durable cancer regression off-treatment and effective reinduction therapy with an anti-PD-1 antibody. Clin Cancer Res. 2013;19:462-8.
2. Le DT, Uram JN, Wang H, et al. PD-1 blockade in tumors with mismatch-repair deficiency. N Engl J Med. 2015;372:2509-20.
3. KEYTRUDA (pembrolizumab) [package insert]. Merck Sharp & Dohme Corp. Whitehouse Station, NJ. https://www.keytruda.com/. Accessed March 12, 2019.
4. Pritchard CC, Morrissey C, Kumar A, et al. Complex MSH2 and MSH6 mutations in hypermutated microsatellite unstable advanced prostate cancer. Nat Commun. 2014;5:4988.
5. Guedes LB, Antonarakis ES, Schweizer MT, et al. MSH2 loss in primary prostate cancer. Clin Cancer Res. 2017;23:6863-74.
6. Smits M, van der Doelen MJ, Westdorp H, et al. Immunological and genomic correlates of response to anti-PD1 checkpoint therapy in mismatch proficient and deficient patients with metastasized castration resistant prostate cancer. J Clin Oncol. 2018;36(suppl):abstr 248.
7. National Comprehensive Cancer Network. NCCN Guidelines, prostate cancer. Version 2.2018. https://www.nccn.org/professionals/physician_gls/default.aspx
8. Castro E, Mateo J, Olmos D, de Bono JS. Targeting DNA repair: the role of PARP inhibition in the treatment of castration-resistant prostate cancer. Cancer J. 2016;22:353-6.
9. Eeles R, Goh C, Castro E, et al. The genetic epidemiology of prostate cancer and its clinical implications. Nat Rev Urol. 2014;11:18-31.
10. Pritchard CC, Mateo J, Walsh MF, et al. Inherited DNA-repair gene mutations in men with metastatic prostate cancer. N Engl J Med. 2016;375:443-53.
11. Castro E, Goh C, Olmos D, et al. Germline BRCA mutations are associated with higher risk of nodal involvement, distant metastasis, and poor survival outcomes in prostate cancer. J Clin Oncol. 2013;31:1748-57.
12. Castro E, Goh C, Leongamornlert D, et al. Effect of BRCA mutations on metastatic relapse and cause-specific survival after radical treatment for localised prostate cancer. Eur Urol. 2015;68:186-93.
13. Hoeijmakers JH. DNA damage, aging, and cancer. N Engl J Med. 2009;361:1475-85.
14. Murai J, Huang SY, Das BB, et al. Trapping of PARP1 and PARP2 by clinical PARP inhibitors. Cancer Res. 2012;72:5588-99.
15. Farmer H, McCabe N, Lord CJ, et al. Targeting the DNA repair defect in BRCA mutant cells as a therapeutic strategy. Nature. 2005;434:917-21.
16. McCabe N, Turner NC, Lord CJ, et al. Deficiency in the repair of DNA damage by homologous recombination and sensitivity to poly(ADP-ribose) polymerase inhibition. Cancer Res. 2006;66:8109-15.
17. Fong PC, Boss DS, Yap TA, et al. Inhibition of poly(ADP-ribose) polymerase in tumors from BRCA mutation carriers. N Engl J Med. 2009;361:123-34.
18. Mateo J, Carreira S, Sandhu S, et al. DNA-repair defects and olaparib in metastatic prostate cancer. N Engl J Med. 2015;373:1697-708.
19. Balmaña J, Tung NM, Isakoff SJ, et al. Phase I trial of olaparib in combination with cisplatin for the treatment of patients with advanced breast, ovarian and other solid tumors. Ann Oncol. 2014;25:1656-63.
20. Schiewer MJ, Goodwin JF, Han S, et al. Dual roles of PARP-1 promote cancer growth and progression. Cancer Discov. 2012;2:1134-49.
21. Ta HQ, Gioeli D. The convergence of DNA damage checkpoint pathways and androgen receptor signaling in prostate cancer. Endocr Relat Cancer. 2014;21:R395-R407.
22. Brenner JC, Ateeq B, Li Y, et al. Mechanistic rationale for inhibition of poly(ADP-ribose) polymerase in ETS gene fusion-positive prostate cancer. Cancer Cell. 2011;19:664-78.
23. Asim M, Tarish F, Zecchini HI, et al. Synthetic lethality between androgen receptor signalling and the PARP pathway in prostate cancer. Nat Commun. 2017;8:374.
24. Hussain M, Daignault S, Twardowski P, et al. Abiraterone + prednisone (Abi) +/- veliparib (Vel) for patients (pts) with metastatic castration-resistant prostate cancer (CRPC): NCI 9012 updated clinical and genomics data. J Clin Oncol. 2017;35(suppl):abstr 5001.
25. Clarke N, Wiechno P, Alekseev B, et al. Olaparib combined with abiraterone in patients with metastatic castration-resistant prostate cancer: a randomised, double-blind, placebo-controlled, phase 2 trial. Lancet Oncol. 2018;19:975-86.
26. Jackaman C, Majewski D, Fox SA, et al. Chemotherapy broadens the range of tumor antigens seen by cytotoxic CD8(+) T cells in vivo. Cancer Immunol Immunother. 2012;61:2343-56.
27. Brown JS, Sundar R, Lopez J. Combining DNA damaging therapeutics with immunotherapy: more haste, less speed. Br J Cancer. 2018;118:312-24.
28. Karzai F, Madan RA, Owens H, et al. A phase 2 study of olaparib and durvalumab in metastatic castrate-resistant prostate cancer (mCRPC) in an unselected population. J Clin Oncol. 2018;36(suppl):abstr 163.
29. Hovelson DH, Tomlins SA. The role of next-generation sequencing in castration-resistant prostate cancer treatment. Cancer J. 2016;22:357-61.
30. Robinson D, Van Allen EM, Wu YM, et al. Integrative clinical genomics of advanced prostate cancer. Cell. 2015;161:1215-28.
31. Grasso CS, Wu YM, Robinson DR, et al. The mutational landscape of lethal castration-resistant prostate cancer. Nature. 2012;487:239-43.
32. Beltran H, Yelensky R, Frampton GM, et al. Targeted next-generation sequencing of advanced prostate cancer identifies potential therapeutic targets and disease heterogeneity. Eur Urol. 2013;63:920-6.
33. Hovelson DH, McDaniel AS, Cani AK, et al. Development and validation of a scalable next-generation sequencing system for assessing relevant somatic variants in solid tumors. Neoplasia. 2015;17:385-99.
34. Attard G, Parker C, Eeles RA, et al. Prostate cancer. Lancet. 2016;387:70-82.
