Neuroendocrine (Small-Cell) Carcinomas: Why They Teach Us Essential Lessons About Prostate Cancer
Aggressive variants of prostate cancer often take the form of neuroendocrine or small-cell carcinomas, which frequently lack androgen receptor expression and respond poorly to hormonal therapies.
Figure 1: Frequent Small-Cell Prostate Carcinoma Presentations
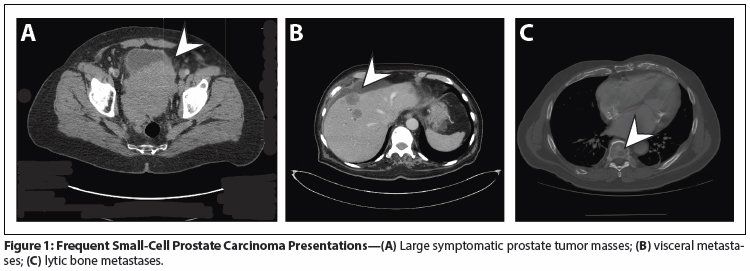
Table 1: Common Molecular Alterations Found in Small-Cell Prostate Carcinomas
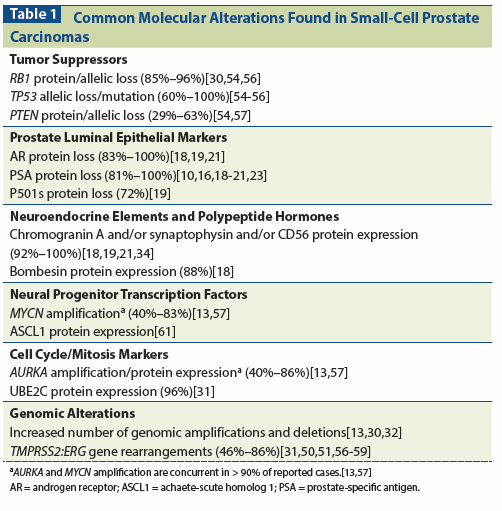
Figure 2: Pathology of Mixed Small-Cell Carcinoma and Adenocarcinoma, Acinar Type vs Small-Cell Carcinoma, Intermediate-Cell Type
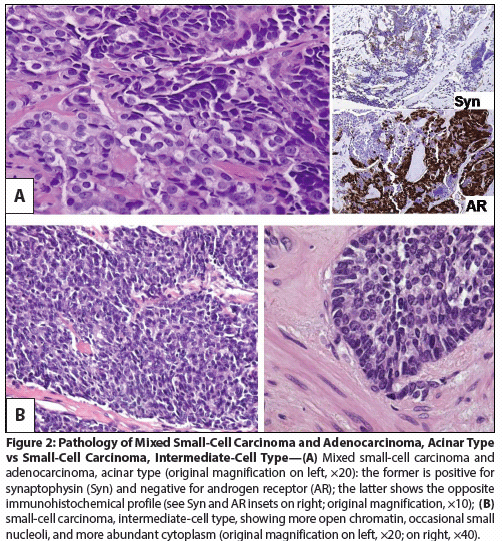
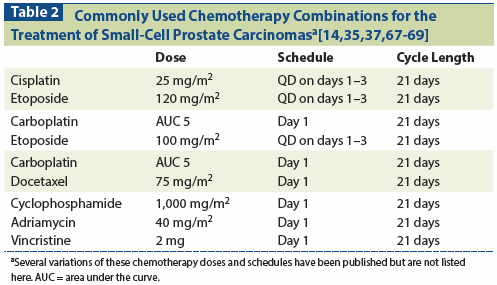
Table 2: Commonly Used Chemotherapy Combinations for the Treatment of Small-Cell Prostate Carcinomasa[14,35,37,67-69]
Atypical clinical features in men with prostate cancer-such as clinical evidence of disease progression in the absence of a proportional increase in serum prostate-specific antigen level, bulky symptomatic tumor masses, exclusive visceral metastases, or a predominance of lytic bone metastases-should alert the clinician that an aggressive prostate cancer variant is present or emerging. Aggressive variants of prostate cancer often take the form of neuroendocrine or small-cell carcinomas, which frequently lack androgen receptor expression and respond poorly to hormonal therapies. Indeed, the finding of neuroendocrine or small-cell prostate carcinoma indicates the need for multimodality treatments that incorporate early combination chemotherapy and locoregional control of bulky tumor deposits, including untreated or recurrent primaries. As we learn to recognize this prostate cancer variant more often, we are reminded that not all prostate cancers share the same biology and that the androgen receptor is not the sole driver of this disease.
The Blast Crisis of Prostate Cancer
A 76-year-old white male with a history of hypertension, atrial fibrillation, gout, cerebrovascular accident, sleep apnea, rosacea, eczema, and longstanding nocturia and urinary frequency was found to have a palpable apical prostatic nodule and a prostate-specific antigen (PSA) level of 12.83 ng/mL in June 2007. Biopsies revealed Gleason 4+5 adenocarcinoma in 7 of 12 cores. Imaging showed a 3.7-cm left iliac lymph node and an L1 blastic lesion. The patient began androgen deprivation therapy in August 2007. In January 2010, he developed rapidly increasing rib and lower back pain. Serum PSA level was 0.8 ng/mL, carcinoembryonic antigen (CEA) level was 8.4 ng/mL, and chromogranin A level was 347 ng/mL. Repeat imaging showed lytic bone lesions, as well as a 3-cm soft-tissue mass in the pelvis. A biopsy of a T8 vertebral metastasis showed metastatic undifferentiated small-cell carcinoma. Weekly carboplatin and docetaxel, beginning in January 2010, resulted in marked pain relief. In July 2010, he developed left lower extremity lymphedema, attributed to progression of his left pelvic mass. He began weekly cisplatin and etoposide, followed by radiation therapy to the left pelvic lymph nodes to a dose of 50 Gy. In November 2010, he began a course of cyclophosphamide, doxorubicin, and vincristine but presented in December 2010 with worsening back pain; he received radiation therapy to T2–T11 and passed away from disease progression in February 2011.
Although rare at the time of initial diagnosis, small-cell carcinomas of the prostate are now increasingly recognized when they arise during the castration-resistant progression of a previously diagnosed prostate adenocarcinoma. Autopsy series published in the last 2 decades describe their presence in 10% to 20% of men dying of castration-resistant prostate cancer,[1-3] and approximately two-thirds of the reported patients have a prior history of typical prostatic adenocarcinoma.[4-6] Whether the prevalence of small-cell carcinomas will change as a result of more effective androgen receptor (AR) targeting is unknown. To date, there is no evidence that the clinical behavior, molecular features, and response to therapy are different in primary (present at initial diagnosis) vs secondary (emerging in the background of a previously diagnosed, seemingly typical prostatic adenocarcinoma) small-cell prostate carcinomas, nor in patients described as having pure small-cell prostate carcinoma vs those in whom small-cell carcinoma is mixed with prostate adenocarcinoma components.[7,8]
Not unlike the terminal blast crises of chronic myelogenous leukemia (CML), the presence of small-cell carcinoma morphology is associated with symptoms of accelerated disease progression and a median survival of 9 to 17 months.[4,5,8-10] Patients frequently present with rapid onset of pain or lower urinary tract obstructive symptoms, and the diagnosis is often made upon transurethral resection of the prostate.[4,5,11] The presence of large pelvic masses often causes intense suffering, as it typically results in pelvic pain, hematuria, hematochezia, and tenesmus at some point during the disease progression (Figure 1). Lytic bone metastases, exclusive visceral metastases, unusual sites of metastases (such as the omentum, penis, skin, pleura, or brain), and symptomatic progression in the setting of low or stable PSA levels[12] are common, as are weight loss, anorexia, weakness, malaise, and other systemic complaints.[4,5,10,11] A number of reports describe an association of small-cell carcinoma with paraneoplastic syndromes,[8,13-23] although their incidence may be overestimated in the literature due to a detection and publication bias. Nonetheless, signs and symptoms consistent with a paraneoplastic syndrome, as well as the onset of the clinical features previously described in men with a prior history of prostate carcinoma, or the evidence of clinical or radiological progression in the absence of a concomitant PSA rise, should alert the clinician that a small-cell prostate carcinoma may be emerging.
To date, there are no serum markers that specifically signal the emergence of small-cell prostate carcinomas. Increased levels of a number of markers can be found in the serum of affected men, including chromogranin A, neuron-specific enolase (NSE), CEA, lactate dehydrogenase, cancer antigen (CA)19-9, CA15-3, and CA125.[2,5,10,12,24,25] Serum levels of hormones such as glucagon, parathyroid hormone (PTH), bombesin, adrenocorticotropic hormone (ACTH), calcitonin, and somatostatin can also be elevated in the absence of a paraneoplastic clinical syndrome.[9,26-28] While anecdotally it appears that the serum levels of these markers may correlate with tumor burden and serve as a measure of response to therapy, this has not been formally tested. It should also be noted that small-cell prostate carcinoma is often mixed with typical prostate adenocarcinoma components, and thus, PSA and prostatic acid phosphatase (PAP) serum levels can be elevated as well.[5,8-11,29]
Also reminiscent of the blast crises of CML, several studies in both patient-tumor–derived xenografts and patient-tumor samples have shown that small-cell prostate carcinomas commonly contain alterations in TP53 (with aberrant nuclear accumulation and mutations detected in 60% to 100% of cases[30-35]), RB1 (with protein and copy number losses found in 85% to 96% of cases[30,35,36]), and PTEN,[30,34,35,37] as well as an increase in the frequency of copy number alterations compared with what is typically seen in castration-resistant prostate carcinomas.[7,36] What role these molecular alterations play in the pathogenesis of small-cell prostate carcinomas remains to be determined.
Biological Clues From the Clinic: Hormone Resistance and Paraneoplastic Syndromes
A 49-year-old white male presented with symptoms of tenesmus, urinary obstruction, fatigue, and weight loss in May 2008. Digital rectal exam revealed a 100-mL nodular firm prostate with seminal vesicle and rectal wall invasion. Biopsies showed poorly differentiated neuroendocrine carcinoma with no uninvolved prostatic tissue. The tumor cells were focally positive for chromogranin, diffusely positive for synaptophysin and CD56, dot-like positive for pan-cytokeratin, and negative for prosaposin (PSAP) and PSA. A positron emission tomography (PET)/CT scan revealed intense uptake within the large lobulated prostate gland, as well as findings consistent with metastatic disease in the lungs, left adrenal gland, and retroperitoneal and pelvic lymph nodes. Serum PSA level was 2.0 ng/mL, CEA level was 979.4 ng/mL, and chromogranin A level, 11.1 ng/mL. The patient received an injection of leuprolide and 2 cycles of carboplatin and docetaxel on a clinical trial, starting in August 2008; he had a minor symptomatic response to this regimen, followed by rapid disease progression. He then received 4 cycles of cisplatin and etoposide, starting in October 2008, to which he had a partial response, with marked improvement in symptoms. However, he soon developed brain metastases that required whole-brain radiation; he also developed progressive pelvic pain and severe symptoms of rectal and urinary obstruction. He then had a partial response to cyclophosphamide, doxorubicin, and vincristine, which was consolidated with 50 Gy of palliative radiation to his prostate and pelvic lymph nodes, completed in June 2009. His disease progressed shortly thereafter, with symptomatic penile metastases, and he succumbed in September 2009.
Based on a review of the clinical course of the 50 small-cell carcinomas reported in the literature by 1992, Moore et al[38] concluded that small-cell prostate carcinomas are hormonally resistant tumors and should be treated with immediate chemotherapy. Indeed, ensuing immunohistochemical studies showed that small-cell prostate carcinomas commonly lack AR expression, as well as other markers of prostatic luminal differentiation, such as PSA, PSAP, prostate-specific membrane antigen (PSMA), and P501s (prostein).[1,2,6,8,12,17,21,24-26,29,32-34,39-44] However, because small-cell carcinoma of the prostate is often admixed with high-grade AR-positive adenocarcinoma components, and because, in at least one preclinical model, its growth was found to be modestly increased in the presence of androgen (despite the absence of AR expression),[32] the general practice has been to incorporate surgical or chemical castration into its management.
Interestingly, the very first cases of small-cell carcinomas of the prostate were identified over 50 years ago, on the basis of their association with syndromes of ectopic ACTH production.[13-18] Early studies included descriptions of their electron microscopy appearance, which frequently featured small-size, dense-core, roughly circular neurosecretory-type granules characteristic of amine precursor uptake and decarboxylation cells.[1,16,44-46] Later reports described associations with various paraneoplastic syndromes, including Lambert-Eaton myasthenic syndrome,[21] inappropriate secretion of antidiurietic hormone,[8,22,23] thyroxine intoxication,[8] and hypercalcemia.[19,20] Immunohistochemical studies demonstrated frequent staining for various neuroendocrine elements and polypeptide hormones (reflecting the presence of cytoplasmic neurosecretory granules), such as NSE, chromogranin, synaptophysin, CD56, calcitonin, PTH, and serotonin (Table 1). A vast number of preclinical studies have since reported that diverse stressors (such as androgen depletion) result in the upregulation of neuroendocrine elements in prostate cancer cells, which in turn modify their growth and invasive properties.[47,48] More recently, transcription factors involved in neural development, such as achaete-scute homolog 1 (ASCL1), neurogenic differentiation 1, and MYCN, which regulate the expression of neuroendocrine elements, have also been found to be overexpressed in tumors with neuroendocrine differentiation.[7,34,49,50] Further upstream, the expression of RE1-silencing transcription factor (REST), a master repressor of neuronal differentiation,[51] has been found to be decreased in small-cell carcinomas of the prostate.[52] Thus, it appears that as prostate tumors dedifferentiate, a neural development program takes the place of the prostatic epithelial luminal differentiation program. However, because the expression of neuroendocrine elements is shared by tumors of diverse biology and prognosis, the pathogenic relevance of the neural/neuroendocrine features remains to be confirmed.
Another clinically evident feature of small-cell prostate carcinomas-their high proliferative capacity-is reflected in a high level of Ki-67 staining and expression of a large number of genes involved in the cell cycle and mitosis, including AURKA, AURKB, PLK1, and UBE2C.[7,36,52] Interestingly, Beltran et al[7] found concordant AURKA and MYCN amplification in approximately 40% of small-cell prostate carcinomas, and Svensson et al[53] observed derepression of cell-cycle genes in response to REST knockdown, suggesting a link between the neural development program and the altered mitotic program.
Biology Beyond Morphology
In 2008, a 60-year-old white male with bilateral hearing deficits developed symptoms of urinary obstruction, intermittent hematuria, and hematospermia. In January 2009, a digital rectal examination revealed a markedly enlarged, firm, nodular prostate with extension to bilateral seminal vesicles. Serum PSA level was 12.5 ng/mL. Biopsies revealed Gleason 4+5, 5+4, and 5+5 prostate carcinoma in 12 of 12 cores. Imaging revealed bilateral pelvic lymphadenopathy. The patient began androgen deprivation therapy in March 2009. In September 2009, his PSA level was < 0.1 ng/mL, but he complained of hematospermia, and his examination findings were suggestive of disease progression. Prostate biopsies revealed high-grade adenocarcinoma in all cores. The tumor responded to carboplatin and docetaxel, and in March 2010 he underwent a radical prostatectomy, yielding a pT4 N1 (3 of 35 lymph nodes), Gleason 5+4 adenocarcinoma of the prostate with neuroendocrine differentiation. The margins of resection were free of tumor. The tumor did not display evident effects of therapy. Immunohistochemical stains demonstrated areas of the tumor that were negative for PSA and PAP and positive for neuroendocrine markers (synaptophysin diffuse, chromogranin focal), but these areas did not have significantly different cytologic features from areas that stained positive for PSA and PAP. In January 2011, his PSA level remained 0.2 ng/mL, but an exam and scans showed thickening at the right bladder base; biopsies once again showed high-grade adenocarcinoma. He began weekly doxorubicin and paclitaxel, followed by cyclophosphamide, vincristine, and dexamethasone; salvage radiation therapy was completed in July 2011. In May 2012, his PSA level was 2.4 ng/mL, CEA level was 2.7 ng/mL, and chromogranin A level, 154 ng/mL; CT showed new liver lesions, as well as progression of lymphadenopathy and a presacral mass inseparable from the rectum. Bone scan remained negative. A liver biopsy showed poorly differentiated carcinoma focally positive for PAP and prostein and negative for PSA. Scattered neoplastic cells were positive for synaptophysin and chromogranin. He did not respond to carboplatin and etoposide, but developed rectal obstruction requiring a diverting colostomy. A biopsy of the locally recurrent mass was read locally as showing metastatic adenocarcinoma of prostatic origin. He died in June 2013 from disease progression.
The diagnosis of small-cell carcinomas of the prostate is a histologic one, based on the morphologic criteria established for small-cell carcinomas of the lung and defined in the 1999 World Health Organization classification criteria for pulmonary neoplasms. These criteria include a proliferation of small cells (< 4 lymphocytes in diameter) with scant cytoplasm, ill-defined borders, finely granular “salt and pepper” chromatin, absent or inconspicuous nucleoli, frequent nuclear molding, and a high mitotic count (Figure 2).[4,29,41,54] Necrosis and perineural invasion are frequently found.[29]
It is not uncommon for pathologists to stop short of diagnosing small-cell prostate carcinoma because the morphologic criteria are not fully met, even though poorly differentiated and neuroendocrine features are present. Immunohistochemical studies have attempted to shed light on the significance of these morphologic variants. In 1999, Helpap and KÃllermann[40] identified 19 cases of undifferentiated carcinomas of the prostate in which small cells comprised at least 90% of the tumor tissue. In this study, they distinguished between “undifferentiated adenocarcinoma with small cell features” (which stained positive for PSA and AR but negative for NSE, synaptophysin, and chromogranin A) and neuroendocrine small-cell cancers (which stained negative for PSA and AR but positive for NSE, synaptophysin, and chromogranin A). However, this clear-cut distinction has not been apparent in subsequent studies. In 2006, Yao et al[41] reported on immunoreactivity to 23 markers in 18 patients with small-cell prostate carcinoma and 10 patients with Gleason pattern 5b prostate adenocarcinoma (with the latter having larger cells, lower nuclear/cytoplasmic ratios, prominent nucleoli, lack of nuclear molding, and coarse chromatin). In this series, the majority of the small-cell prostate carcinomas stained positive for thyroid transcription factor-1 (TTF-1), CD56, chromogranin A, and synaptophysin or bombesin-but some of the Gleason 5b tumors also stained positive for synaptophysin or bombesin. Virtually all of the Gleason 5b tumors stained positive for PSA, PSAP, or AR, but up to a quarter of the small-cell prostate carcinomas were also positive for one of these markers.[41] In the largest histologic series published to date, Wang and Epstein[29] showed that 14 of 73 (19.2%), 17 of 61 (27.9%), and 15 of 59 (25.4%) prostatic small-cell carcinomas stained positive for PSA, prostein, and PSMA, respectively. They also reported that 33 of 44 (75%), 27 of 32 (84%), 17 of 20 (85%), and 12 of 13 (92%) stained positive for chromogranin, synaptophysin, NSE, and CD56, respectively. One tumor was negative for all three neuroendocrine markers, and two were negative for both chromogranin and synaptophysin. (CD56 was unknown.) Here, the authors noted that 64% of the specimens displayed classic oat-cell morphology but the remainder had more abundant cytoplasm, slightly larger nuclei, and more visible nucleoli, fitting an “intermediate-cell type” morphology previously described. [14,29,44,54] More recently, a third variant, termed “prostate carcinoma with overlapping features of small-cell and acinar adenocarcinoma” has been proposed; this would include tumors for which a distinction between small-cell carcinoma and Gleason pattern 5 adenocarcinoma cannot be made.[54] In these tumors, sheet-like architecture and scant cytoplasm are suggestive of neuroendocrine carcinoma, but nucleoli are more prominent.[54]
To date, there is no evidence that the above variations in morphology are associated with any differences in clinical behavior, response to therapy, or outcome. Furthermore, the practice of obtaining biopsies in patients with prostate cancer who exhibit clinical features characteristic of small-cell prostate carcinomas (such as exclusive visceral disease, lytic bone metastases, bulky tumor masses) led to the realization that these features were also often present even when only adenocarcinoma morphology was present. Moreover, the presence of these features alone appeared to predict for a course and response to therapy similar to that of small-cell prostate carcinomas. Thus, a prospective phase II clinical trial in men with prostate cancers that behaved like small-cell prostate carcinomas, regardless of the actual histologic diagnosis, was conducted. The experience from this trial lent support to the hypothesis that these clinically defined aggressive variant prostate carcinomas also shared small-cell carcinomas’ responsiveness to combination chemotherapy and thus should be managed in a similar manner.[55] Ongoing studies suggest that these clinically defined, morphologically heterogeneous, aggressive variant prostate carcinomas also share molecular features with small-cell prostate carcinomas, such as loss of prostatic luminal epithelial markers; increase in cell-cycle, mitotic, and neural development markers; and alterations in RB1, TP53, and PTEN.[56] These observations imply that a “small-cell program” may be present in a larger proportion of prostate cancers than previously thought.
The Importance of Immediate Chemotherapy and Locoregional Control
A 49-year-old white male presented in August 2008 with bilateral rib cage pain, decreased urinary stream, and perineal pain. His PSA level was 137 ng/mL. In October 2008, a bone scan revealed multiple bone metastases, and CT showed an enlarged prostate with nodularity around the rectum and extensive lymphadenopathy. Prostate biopsies showed Gleason 5+5 prostate carcinoma, with some areas suspicious for small-cell carcinoma. He developed bilateral lower extremity motor and sensory deficits, and MRI showed T5–T7 metastatic disease with cord compression. He received radiation therapy and began androgen deprivation therapy, but by December 2008, his PSA level was 181.6 ng/mL, his testosterone level was 121 ng/mL, and prostate biopsies showed high-grade carcinoma without evidence of small-cell or neuroendocrine differentiation. He resumed androgen deprivation therapy; his PSA level nadired at 0.6 ng/mL in March 2009, but by July it was up to 4.6 ng/mL. Repeat prostate biopsies now showed treated adenocarcinoma and small-cell carcinoma elements. The serum CEA level was < 1.0 ng/mL, and chromogranin A level was 103 ng/mL. He received 5 cycles of carboplatin and docetaxel on a clinical trial completed in October 2009, but by February 2010, he had developed worsening urinary symptoms and evidence of disease progression. After 4 cycles of cisplatin and etoposide, in June 2010, cystoscopic evaluation revealed tumor involving the trigone and bladder neck. He was treated with various systemic regimens, including cyclophosphamide, doxorubicin, and vincristine; cabazitaxel; and a phase I clinical trial of lenalidomide and bevacizumab-but showed no improvement with any of these. By February 2011, he had developed severe pelvic pain and complete urinary obstruction, requiring bilateral percutaneous nephrostomy tubes for palliation. He passed away from disease progression in July 2011.
The mainstay of therapy for small-cell prostate carcinomas is combination chemotherapy. In general, the duration of response is short, but it frequently provides marked palliation of symptoms and occasionally results in durable remissions.[12,17,25,27,57-61] Multiple case reports and series have described the response of small-cell prostate carcinomas to various chemotherapy combinations, but only two prospective, single-arm clinical trials of this disease entity have been published.[9,55]
In the first trial, the goal was to explore the hypothesis that the combination of doxorubicin, etoposide, and cisplatin would improve the response rate of these cancers over what had been observed historically with etoposide and cisplatin alone. Of the 36 patients who were assessable for response, 22 (61%) achieved a partial response in measurable disease. Notably, 21 of 25 symptomatic patients (84%) experienced pain reduction, with a concomitant decrease in their daily opioid analgesic requirement. Median time to progression and overall survival were 5.8 months (95% confidence interval [CI], 4.1–6.9) and 10.5 months (95% CI, 7.5–14.3), respectively. However, toxicity was significant, and the authors concluded that the addition of doxorubicin to etoposide and cisplatin was not an improvement.[9]
In the second trial,[55] men with castration-resistant prostate cancers that behaved clinically like small-cell prostate carcinomas, whether or not they had biopsy-proven small-cell prostate carcinomas, were treated with frontline carboplatin and docetaxel, followed by cisplatin and etoposide at progression. Twenty-nine of 114 eligible patients in this series (25.4%) had histological confirmation of small-cell prostate carcinoma. Of the 27 (23.9%) who received frontline carboplatin and docetaxel on study, 20 (74%) and 14 (51.8%) were free of disease progression after 2 and 4 cycles, respectively. Of the 19 patients who went on to receive second-line cisplatin and etoposide, 13 (68.4%) and 7 (36.8%) were free of disease progression after 2 and 4 cycles, respectively.
Additional chemotherapy regimens frequently reported in the literature include carboplatin with etoposide; and cyclophosphamide, doxorubicin, and vincristine-both of which have been used based on small-cell lung cancer literature (Table 2).[10,11,55,60,62,63] Data that might be used to determine the optimal number of cycles of chemotherapy do not exist. Given the cumulative toxicity of these chemotherapy combinations, the general practice has been to treat with 2 cycles beyond best response (typically between 4 and 8 cycles) and then consider consolidative locoregional efforts to control bulky and symptomatic tumor masses.
Small-cell prostate carcinomas are highly metastatic but also highly invasive and locally aggressive. Local invasion and progression often lead to severe complications (such as renal insufficiency, which limits therapy options), as well as profound suffering during the course of the disease (Figure 1).[5,64] Therefore, when possible, aggressive multimodality treatment of the primary tumor should be incorporated early into the treatment plan, whether or not distant metastatic disease is present.[5,62] In general, it is recommended that patients begin by receiving systemic combination therapy (see Table 2) and, at the time of maximal response, undergo consolidative radiation or surgery. In the absence of data to support one or the other of these two local modalities, the choice between primary radiation and surgery should be individualized based on the bulk of residual tumor, the extent of invasion of neighboring organs, the extent and control of metastatic disease, and the comorbidities and performance status of the patient. Inclusion of pelvic lymph nodes in the treatment plan is not known to improve outcomes but might be considered, based on the anecdotal experience that disease progression in the lymph nodes also frequently leads to morbid complications. If surgery is the primary approach, postsurgical radiation might be considered in selected cases (eg, in the presence of extensive positive margins and limited distant disease). It is noteworthy that recent retrospective series of patients with small-cell prostate carcinomas have reported on the approximately 25% of included patients who presented with nonmetastatic disease. Compared with men who presented with metastatic small-cell prostate carcinomas, the former patients were older (median age, 70/71 years; range, 43–88 years vs median, 63.5/67 years; range, 40–86 years) and had a worse median progression-free survival (5 vs 10 months [95% CI, 8.1–16.1]) and disease-specific survival (12.5 vs 17.1 months [95% CI, 12.1–39.2]).[5,60]
Finally, although Spiess et al[5] reported that 8 of 83 patients with small-cell prostate carcinomas (10%) developed brain metastases, other case reports and series do not describe the brain as a common site of treatment failure. Perhaps with improved management of the systemic disease, brain metastases will become an increasing problem, but to date, there are insufficient data to support the use of prophylactic cranial irradiation in this entity. Nonetheless, surveillance with periodic brain imaging is recommended.
Conclusions
Small-cell or neuroendocrine carcinomas of the prostate have become an increasingly recognized clinical problem that has garnered attention as a truly AR-independent subset of lethal prostate carcinomas, one that is associated with clinical behavior and a response to therapy distinct from those of typical prostate adenocarcinomas. Accordingly, the management of small-cell tumors should be tailored to these differences and should incorporate early combination chemotherapies and aggressive treatment of the primary and bulky tumor masses. The development of genetically engineered mice[65-67] and patient-derived xenografts[32,34,36,39,49] that model this entity, as well as the application of arrays and next-generation sequencing technologies to increasingly available tumor samples, has yielded significant insight into the molecular biology of small-cell prostate cancers.[7,52] The hope is that the increased awareness and clinical knowledge accumulated over the years, along with more recent molecular insights, will translate into effective management strategies that improve outcomes for men affected by this aggressive prostate cancer variant.
Financial Disclosure: The authors have no significant financial interest in or other relationship with the manufacturer of any product or provider of any service mentioned in this article.
References:
1. Turbat-Herrera EA, Herrera GA, Gore I, et al. Neuroendocrine differentiation in prostatic carcinomas. A retrospective autopsy study. Arch Pathol Lab Med. 1988;112:1100-5.
2. Tanaka M, Suzuki Y, Takaoka K, et al. Progression of prostate cancer to neuroendocrine cell tumor. Int J Urol. 2001;8:431-6; discussion 37.
3. Shah RB, Mehra R, Chinnaiyan AM, et al. Androgen-independent prostate cancer is a heterogeneous group of diseases: lessons from a rapid autopsy program. Cancer Res. 2004;64:9209-16.
4. Tetu B, Ro JY, Ayala AG, et al. Small cell carcinoma of the prostate. Part I. A clinicopathologic study of 20 cases. Cancer. 1987;59:1803-9.
5. Spiess PE, Pettaway CA, Vakar-Lopez F, et al. Treatment outcomes of small cell carcinoma of the prostate: a single-center study. Cancer. 2007;110:1729-37.
6. Christopher ME, Seftel AD, Sorenson K, Resnick MI. Small cell carcinoma of the genitourinary tract: an immunohistochemical, electron microscopic and clinicopathological study. J Urol. 1991;146:382-8.
7. Beltran H, Rickman DS, Park K, et al. Molecular characterization of neuroendocrine prostate cancer and identification of new drug targets. Cancer Discov. 2011;1:487-95.
8. Oesterling JE, Hauzeur CG, Farrow GM. Small cell anaplastic carcinoma of the prostate: a clinical, pathological and immunohistological study of 27 patients. J Urol. 1992;147:804-7.
9. Papandreou CN, Daliani DD, Thall PF, et al. Results of a phase II study with doxorubicin, etoposide, and cisplatin in patients with fully characterized small-cell carcinoma of the prostate. J Clin Oncol. 2002;20:3072-80.
10. Amato RJ, Logothetis CJ, Hallinan R, et al. Chemotherapy for small cell carcinoma of prostatic origin. J Urol. 1992;147:935-7.
11. Palmgren JS, Karavadia SS, Wakefield MR. Unusual and underappreciated: small cell carcinoma of the prostate. Semin Oncol. 2007;34:22-9.
12. Sella A, Konichezky M, Flex D, et al. Low PSA metastatic androgen-independent prostate cancer. Eur Urol. 2000;38:250-4.
13. Webster GD, Jr, Touchstone JC, Suzuki M. Adrenocortical hyperplasia occurring with metastatic carcinoma of the prostate: report of a case exhibiting increased urinary aldosterone and glucocorticoid excretion. J Clin Endocrinol Metab. 1959;19:967-79.
14. Newmark SR, Dluhy RG, Bennett AH. Ectopic adrenocorticotropin syndrome with prostatic carcinoma. Urology. 1973;2:666-8.
15. Lovern WJ, Fariss BL, Wettlaufer JN, Hane S. Ectopic ACTH production in disseminated prostatic adenocarcinoma. Urology. 1975;5:817-20.
16. Wenk RE, Bhagavan BS, Levy R, et al. Ectopic ACTH, prostatic oat cell carcinoma, and marked hypernatremia. Cancer. 1977;40:773-8.
17. Adshead F, De Graeff A, Mansi JL, et al. Small cell carcinoma of the prostate: implications for management. Br J Urol. 1991;67:217-8.
18. Nimalasena S, Freeman A, Harland S. Paraneoplastic Cushing’s syndrome in prostate cancer: a difficult management problem. BJU Int. 2008;101:424-7.
19. Mahadevia PS, Ramaswamy A, Greenwald ES, et al. Hypercalcemia in prostatic carcinoma. Report of eight cases. Arch Intern Med. 1983;143:1339-42.
20. Smith DC, Tucker JA, Trump DL. Hypercalcemia and neuroendocrine carcinoma of the prostate: a report of three cases and a review of the literature. J Clin Oncol. 1992;10:499-505.
21. Tetu B, Ro JY, Ayala AG, et al. Small cell carcinoma of prostate associated with myasthenic (Eaton-Lambert) syndrome. Urology. 1989;33:148-52.
22. Sellwood RA, Spencer J, Azzopardi JG, et al. Inappropriate secretion of antidiuretic hormone by carcinoma of the prostate. Br J Surg. 1969;56:933-5.
23. Ghandur-Mnaymneh L, Satterfield S, Block NL. Small cell carcinoma of the prostate gland with inappropriate antidiuretic hormone secretion: morphological, immunohistochemical and clinical expressions. J Urol. 1986;135:1263-6.
24. Miyoshi Y, Uemura H, Kitami K, et al. Neuroendocrine differentiated small cell carcinoma presenting as recurrent prostate cancer after androgen deprivation therapy. BJU Int. 2001;88:982-3.
25. Okada H, Gotoh A, Ogawa T, et al. Two cases of small cell carcinoma of the prostate. Scand J Urol Nephrol. 1996;30:503-8.
26. Hagood PG, Johnson FE, Bedrossian CW, Silverberg AB. Small cell carcinoma of the prostate. Cancer. 1991;67:1046-50.
27. Yashi M, Terauchi F, Nukui A, et al. Small-cell neuroendocrine carcinoma as a variant form of prostate cancer recurrence: a case report and short literature review. Urol Oncol. 2006;24:313-7.
28. Fjellestad-Paulsen A, Abrahamsson PA, Bjartell A, et al. Carcinoma of the prostate with Cushing’s syndrome. A case report with histochemical and chemical demonstration of immunoreactive corticotropin-releasing hormone in plasma and tumoral tissue. Acta Endocrinol (Copenh). 1988;119:506-16.
29. Wang W, Epstein JI. Small cell carcinoma of the prostate. A morphologic and immunohistochemical study of 95 cases. Am J Surg Pathol. 2008;32:65-71.
30. Tan HL, Sood A, Rahimi HA, et al. Rb loss is characteristic of prostatic small cell neuroendocrine carcinoma. Clin Cancer Res. 2014;20:890-903.
31. Chen H, Sun Y, Wu C, et al. Pathogenesis of prostatic small cell carcinoma involves the inactivation of the P53 pathway. Endocr Relat Cancer. 2012;19:321-31.
32. Pinthus JH, Waks T, Schindler DG, et al. WISH-PC2: a unique xenograft model of human prostatic small cell carcinoma. Cancer Res. 2000;60:6563-7.
33. Hansel DE, Nakayama M, Luo J, et al. Shared TP53 gene mutation in morphologically and phenotypically distinct concurrent primary small cell neuroendocrine carcinoma and adenocarcinoma of the prostate. Prostate. 2009;69:603-9.
34. Aparicio A, Tzelepi V, Araujo JC, et al. Neuroendocrine prostate cancer xenografts with large-cell and small-cell features derived from a single patient’s tumor: morphological, immunohistochemical, and gene expression profiles. Prostate. 2011;71:846-56.
35. Lin D, Wyatt AW, Xue H, et al. High fidelity patient-derived xenografts for accelerating prostate cancer discovery and drug development. Cancer Res. 2014;74:1272-83.
36. Tzelepi V, Zhang J, Lu JF, et al. Modeling a lethal prostate cancer variant with small-cell carcinoma features. Clin Cancer Res. 2012;18:666-77.
37. Mosquera JM, Beltran H, Park K, et al. Concurrent AURKA and MYCN gene amplifications are harbingers of lethal treatment-related neuroendocrine prostate cancer. Neoplasia. 2013;15:1-10.
38. Moore SR, Reinberg Y, Zhang G. Small cell carcinoma of prostate: effectiveness of hormonal versus chemotherapy. Urology. 1992;39:411-6.
39. van Haaften-Day C, Raghavan D, Russell P, et al. Xenografted small cell undifferentiated cancer of prostate: possible common origin with prostatic adenocarcinoma. Prostate. 1987;11:271-9.
40. Helpap B, KÃllermann J. Undifferentiated carcinoma of the prostate with small cell features: immunohistochemical subtyping and reflections on histogenesis. Virchows Arch. 1999;434:385-91.
41. Yao JL, Madeb R, Bourne P, et al. Small cell carcinoma of the prostate: an immunohistochemical study. Am J Surg Pathol. 2006;30:705-12.
42. Lotan TL, Gupta NS, Wang W, et al. ERG gene rearrangements are common in prostatic small cell carcinomas. Mod Pathol. 2011;24:820-8.
43. Scheble VJ, Braun M, Beroukhim R, et al. ERG rearrangement is specific to prostate cancer and does not occur in any other common tumor. Mod Pathol. 2010;23:1061-7.
44. Ro JY, Tetu B, Ayala AG, Ordonez NG. Small cell carcinoma of the prostate. II. Immunohistochemical and electron microscopic studies of 18 cases. Cancer. 1987;59:977-82.
45. Vuitch MF, Mendelsohn G. Relationship of ectopic ACTH production to tumor differentiation: a morphologic and immunohistochemical study of prostatic carcinoma with Cushing’s syndrome. Cancer. 1981;47:296-9.
46. Schron DS, Gipson T, Mendelsohn G. The histogenesis of small cell carcinoma of the prostate. An immunohistochemical study. Cancer. 1984;53:2478-80.
47. di Sant’Agnese PA. Neuroendocrine differentiation in prostatic carcinoma: an update on recent developments. Ann Oncol. 2001;12(suppl 2):S135-S140.
48. Terry S, Beltran H. The many faces of neuroendocrine differentiation in prostate cancer progression. Frontier Oncol. 2014;4:60.
49. Clegg N, Ferguson C, True LD, et al. Molecular characterization of prostatic small-cell neuroendocrine carcinoma. Prostate. 2003;55:55-64.
50. Tzelepi V, Maitv S, Zhang J, et al. Differential expression of UBE2C and ASCL1 in neuroendocrine carcinoma and adenocarcinoma of the prostate in xenograft models and human samples. USCAP Annual Meeting; 2011; San Antonio, TX.
51. Majumder S. REST in good times and bad: roles in tumor suppressor and oncogenic activities. Cell Cycle. 2006;5:1929-35.
52. Lapuk AV, Wu C, Wyatt AW, et al. From sequence to molecular pathology, and a mechanism driving the neuroendocrine phenotype in prostate cancer. J Pathol. 2012;227:286-97.
53. Svensson C, Ceder J, Iglesias-Gato D, et al. REST mediates androgen receptor actions on gene repression and predicts early recurrence of prostate cancer. Nucleic Acids Res. 2014;42:999-1015.
54. Epstein JI, Amin MB, Beltran H, et al. Proposed morphologic classification of prostate cancer with neuroendocrine differentiation. Am J Surg Pathol. 2014;38:756-67.
55. Aparicio AM, Harzstark A, Corn PG, et al. Platinum-based chemotherapy for variant castrate-resistant prostate cancer. Clin Cancer Res. 2013;19:3621-30.
56. Aparicio A, Maitv SI, Wang XI, et al. A molecular characterization of the anaplastic prostate carcinomas. American Association for Cancer Research Annual Meeting; April 2013; Washington, DC. Abstr 7587.
57. Slovin SF. Does small-cell phenotype predict the natural history of prostate cancer? A case study in disease behavior. Nat Clin Pract Oncol. 2007;4:551-4.
58. Ciszewski A, Shackleton D, Beer TM. Long-term remission of metastatic small cell carcinoma of the prostate. Urology. 2008;71:546.e3-4.
59. Asmis TR, Reaume MN, Dahrouge S, Malone S. Genitourinary small cell carcinoma: a retrospective review of treatment and survival patterns at The Ottawa Hospital Regional Cancer Center. BJU Int. 2006;97:711-5.
60. Stein ME, Bernstein Z, Abacioglu U, et al. Small cell (neuroendocrine) carcinoma of the prostate: etiology, diagnosis, prognosis, and therapeutic implications--a retrospective study of 30 patients from the rare cancer network. Am J Med Sci. 2008;336:478-88.
61. Hindson DA, Knight LL, Ocker JM. Small-cell carcinoma of prostate. Transient complete remission with chemotherapy. Urology. 1985;26:182-4.
62. Socinski MA, Smit EF, Lorigan P, et al. Phase III study of pemetrexed plus carboplatin compared with etoposide plus carboplatin in chemotherapy-naive patients with extensive-stage small-cell lung cancer. J Clin Oncol. 2009;27:4787-92.
63. von Pawel J, Schiller JH, Shepherd FA, et al. Topotecan versus cyclophosphamide, doxorubicin, and vincristine for the treatment of recurrent small-cell lung cancer. J Clin Oncol. 1999;17:658-67.
64. Rubenstein JH, Katin MJ, Mangano MM, et al. Small cell anaplastic carcinoma of the prostate: seven new cases, review of the literature, and discussion of a therapeutic strategy. Am J Clin Oncol. 1997;20:376-80.
65. Greenberg NM, DeMayo F, Finegold MJ, et al. Prostate cancer in a transgenic mouse. Proc Natl Acad Sci USA. 1995;92:3439-43.
66. Masumori N, Thomas TZ, Chaurand P, et al. A probasin-large T antigen transgenic mouse line develops prostate adenocarcinoma and neuroendocrine carcinoma with metastatic potential. Cancer Res. 2001;61:2239-49.
67. Zhou Z, Flesken-Nikitin A, Corney DC, et al. Synergy of p53 and Rb deficiency in a conditional mouse model for metastatic prostate cancer. Cancer Res. 2006;66:7889-98.