Thromboembolism and Bleeding in Bladder Cancer
Overall, approximately 2% of patients with bladder cancer will experience a venous thromboembolism event, a rate five times higher than that in the overall population; also, such an event results in a threefold increased risk of death in patients with cancer.
Figure 1: The Coagulation Cascade
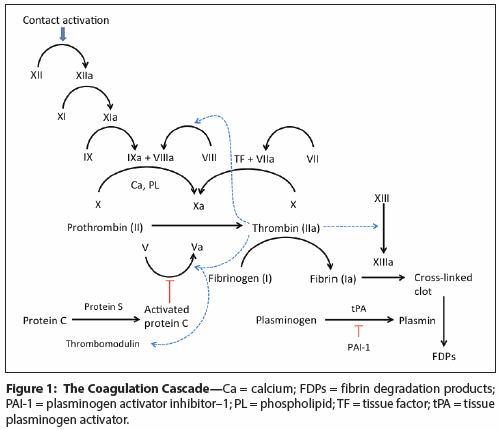
Figure 2: The Fibrinolytic Cascade
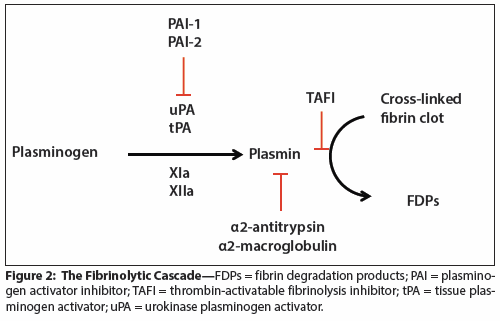
Table 1: Approximate Incidence of Venous Thromboembolism in Bladder Cancer
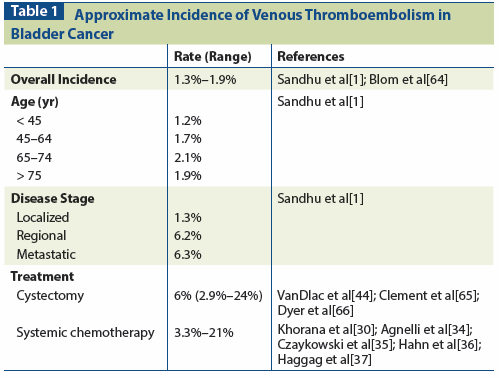
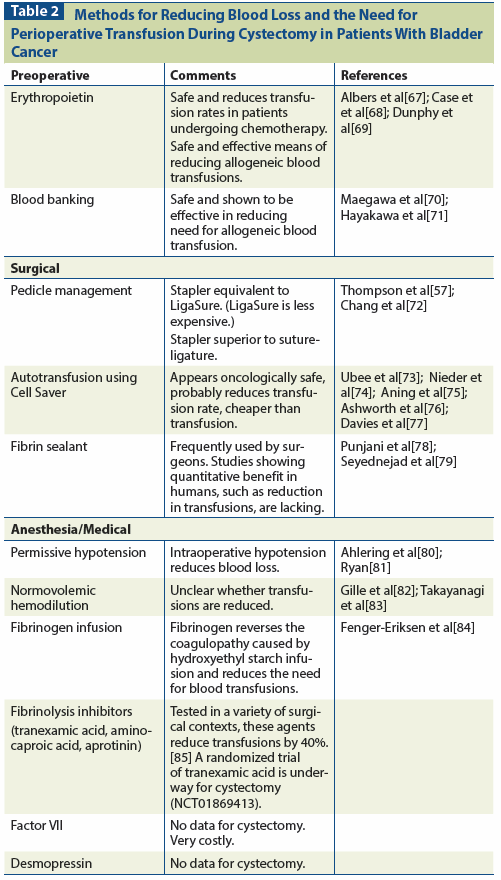
Table 2: Methods for Reducing Blood Loss and the Need for Perioperative Transfusion During Cystectomy in Patients With Bladder Cancer
Bladder cancer is a unique disease process in that clinically significant hemorrhage can occur simultaneously with equally significant aberrant clotting. With hematuria the key presenting symptom of bladder cancer, hemorrhage is generally thought to be a component of the natural history of the disease, and to commonly occur during its treatment. However, as those who regularly treat bladder cancer know, the need to address a predisposition to clotting is also very much part of the treatment paradigm. Physicians must be cognizant of the biochemical changes that confer a propensity for both significant bleeding and clotting occurring simultaneously in their patients. Both of these entities remain important issues, and further study is needed to find ways to mitigate and balance the associated risks. Here, we performed a review of the literature, focusing on the concomitant issues of bleeding and venous thromboembolism in both the pre- and post-operative periods in patients with bladder cancer. We formulated a general management approach with respect to these two processes, and we provide direction for further investigation.
Introduction
Cancer has long been recognized as a risk factor for thrombotic events. While venous thromboembolism (VTE, which includes pulmonary embolism [PE] and deep vein thrombosis [DVT]) is clearly the most common form of malignancy-associated thrombosis, arterial thrombosis and systemic thrombotic syndromes (eg, disseminated intravascular coagulation [DIC]) can also occur. Overall, approximately 2% of patients with bladder cancer (BCa) will experience a VTE event, a rate five times higher than that in the overall population; also, such an event results in a threefold increased risk of death in patients with cancer.[1-3]
It is perhaps unusual that BCa is also strongly associated with bleeding phenomena. For example, hematuria is present in virtually all patients with bladder tumors and is the most common presenting symptom of BCa. Likewise, anemia is present in many patients with BCa, and the most common complication of radical cystectomy is anemia requiring blood transfusion.[4] Physicians who manage BCa are therefore commonly faced with clinical scenarios in which bleeding and clotting risks must be balanced. In this article we will review how BCa dysregulates hemostasis, describe the important clinical sequelae of this dysregulation, and discuss some strategies for clinical management of these sequelae.
Physiologic Coagulation and Fibrinolysis
Damaged blood vessels seal themselves through the natural process of coagulation that results in fibrin clot formation. The normal coagulation cascade, depicted in Figure 1, comprises three pathways: (1) the intrinsic contact activation pathway; (2) the extrinsic tissue factor pathway; and (3) the final common pathway, where the intrinsic and extrinsic pathways converge.
Normally, the extrinsic tissue factor pathway is the primary pathway for the initiation of coagulation and is measured by the prothrombin time (PT) and the related international normalized ratio (INR). Tissue factor (TF, factor III) is a cell surface protein found on leukocytes and subendothelial cells (eg, fibroblasts) and is the only protein in the clotting cascade for which an inherited congenital deficiency has not been found. With blood vessel injury, cells in the subendothelial space become exposed to circulating plasma proteins, including the factor VII zymogen, an inactive protease and the most abundant coagulation factor found in the blood. Factor VII binds TF, and together they become an active serine protease complex known as extrinsic tenase, which activates factors IX and X. Tissue factor pathway inhibitor (TFPI) inhibits both the VIIa-TF complex and Xa, and is therefore an important physiologic mechanism for regulating the extrinsic tissue factor pathway.
The intrinsic contact activation pathway, measured by the activated partial thromboplastin time (aPTT), is activated when blood vessel injury spills plasma proteins onto subendothelial collagen fibers. Several coagulation factors—including factor XII (Hageman factor), high-molecular weight kininogen (HMWK), and prekallikrein—bind to the collagen fibers and become activated. Factor XIIa then activates factor XI, which in turn activates factor IX (Christmas factor). In the presence of calcium and membrane phospholipids, factor IXa binds to factor VIIIa to form the intrinsic tenase complex. Factor IXa is physiologically inhibited by anti-thrombin (AT), while factor VIIIa is inhibited by activated protein C (APC). Although factor XII is used in vitro to activate coagulation, in vivo its deficiency is asymptomatic. Deficiencies of factors VIII, IX, and XI lead to hemophilias A, B, and C, respectively.
The intrinsic and extrinsic pathways converge on the common coagulation pathway since both produce a "tenase" that activates factor X. Factor Xa and factor Va join to form the prothrombinase complex that converts prothrombin (factor II) to thrombin. Thrombin then cleaves the soluble fibrinogen (factor I) into insoluble fibrin strands that are then covalently cross-linked by factor XIIIa to form a blood clot. Physiologic inactivation of the common coagulation pathway occurs by APC inhibition of factor Va, and by AT inhibition of factor Xa and thrombin. Factor V deficiency causes parahemophilia, while certain point mutations in the factor V gene lead to a hypercoagulable state called factor V Leiden. Whereas congenital deficiencies of factor X, prothrombin, or fibrinogen are exceedingly rare, acquired deficiencies of factor X occur with hemodilution (eg, blood loss), consumptive pathologies (eg, sepsis, trauma, DIC), and liver disease.
The last component of physiologic coagulation is fibrinolysis, the process that prevents overabundant clotting and removes clots once healing has occurred. Soon after the coagulation cascade has begun, the fibrinolytic cascade (Figure 2) is activated to eventually break down the clot once the vessel is repaired. The key enzyme controlling fibrinolysis is plasmin, which is activated from plasminogen by tissue plasminogen activator (tPA), urokinase plasminogen activator (uPA), and factors XIa and XIIa. The most important plasminogen activator is tPA, which is slowly released by the endothelium of the injured blood vessel. Once active, plasmin cleaves the fibrin clot into fibrin degradation products (FDPs), the most clinically important of which is the D-dimer. Fibrinolysis is regulated by several mechanisms. First, uPA and tPA are inhibited by the plasminogen activator inhibitors (PAI-1 and PAI-2). Second, plasmin itself is inhibited by the plasma proteins α2-antitrypsin and α2-macroglobulin. Lastly, the fibrin clot is protected by thrombin-activatable fibrinolysis inhibitor (TAFI, also known as carboxypeptidase U), an enzyme that is activated by thrombin and that removes from the fibrin strands the C-terminal residues that are required for binding of plasminogen to the clot.
Alterations in Coagulation and Fibrinolysis in Bladder Cancer
It has been recognized for decades that abnormalities in coagulation and fibrinolysis are present in BCa.[5-7] These abnormalities have been exploited as both diagnostic and prognostic biomarkers, and for therapeutic targeting. Note that these molecular alterations are both pro-thrombotic and pro-fibrinolytic.
Tissue factor (TF)
TF controls the extrinsic coagulation pathway but also has biologic effects that promote angiogenesis and metastasis. Urinary and serum TF levels are elevated in patients with BCa.[8-10] Importantly, TF is overexpressed in approximately 75% of muscle-invasive BCas and is associated with a threefold increased risk of death.[11]
Urokinase plasminogen activator (uPA)
Although its primary role is to activate fibrinolysis, uPA also plays a role in BCa. First, uPA and its receptor are produced by cultured BCa cells and facilitate their invasiveness.[12] Second, uPA and its RNA are elevated in the urine of BCa patients.[13,14] Third, uPA is overexpressed in BCa and associated with a higher recurrence rate and worse survival.[15,16] Interestingly, intravesical uPA has been used (ineffectively) in combination with doxorubicin and thioTEPA as intravesical treatment for BCa.[17-19] Drugs that target uPA and its receptor (uPAR) are being developed.[20]
Plasminogen activator inhibitor-1 (PAI-1)
In addition to inhibiting fibrinolysis, PAI-1 (also known as SERPINE1) is involved in angiogenesis, cell migration, and cell adhesion. It is overexpressed in BCa and its levels in urine and serum appear to increase with tumor stage.[21,22] The presence of even a single cell expressing PAI-1 has been shown to increase BCa-specific mortality 4.5-fold.[22] Targeting PAI-1 was shown to be an effective anticancer therapy in a BCa xenograft model.[23]
Fibrinogen and fibrinogen degradation products (FDPs)
FDPs are elevated in the urine of patients with BCa and are indicative of increased fibrinolysis.[24-26] Intravesical fibrinogen has been administered with the bacillus Calmette Guérin (BCG) vaccine in an attempt to improve the efficacy of the BCG.[27]
Venous Thromboembolism in Bladder Cancer
VTE occurs in 1% to 2% of BCa patients within 2 years of their initial diagnosis, which is a rate 5-fold higher than that of the overall population.[1] This rate is markedly affected by patient factors such as age, disease stage, and type of cancer treatment (Table 1). Importantly, the occurrence of VTE is a prognostic factor-independent of age, BCa stage, and comorbidity-that triples the mortality risk in patients with BCa.[1]
Several risk-assessment tools are available to help predict the risk of VTE. For cancer patients, the Khorana score can be used to predict the probability of VTE and is currently recommended for use in guidelines from the American Society of Clinical Oncology (ASCO), the European Society for Medical Oncology (ESMO), and the National Comprehensive Cancer Network (NCCN).[28] The Khorana score ranges from 0 to 7 points and the cumulative 6-month probability of VTE is approximately 1% for patients at the lowest risk of VTE (a score of 0), 5% for low- to intermediate-risk patients (a score of 1), 10% for intermediate-risk patients (a score of 2), and 20% for high-risk patients (a score ≥ 3). The Caprini score, another tool for predicting VTE, is also applicable to patients without cancer; it is more useful for perioperative risk assessment and is recommended in the 2012 American College of Chest Physicians (ACCP) VTE guidelines.[29]
Bladder cancer is one of several cancer types for which patients are considered to be at high risk of developing VTE.[30] Sandhu et al produced a study of nearly 25,000 patients with BCa that revealed a 2-year cumulative incidence of VTE of 1.9%.[1] The highest incidence was within the first 6 months following diagnosis. Risk of VTE was increased by the presence of metastatic disease at the time of diagnosis, with a 15.3% incidence of VTE in the first 6 months. The authors found, however, that cystectomy conferred a 2.1-fold increase in the risk of developing VTE compared with patients who did not undergo surgery. This is in stark contrast to other major surgically treated malignancies such as breast, lung, and colorectal cancer, in which the risk of VTE is decreased following surgical treatment.[1,31-33] A limitation in Sandhu's study is that those undergoing systemic chemotherapy or using chronic anticoagulation were not identified for subgroup analysis. Some potential reasons for the increased risk of VTE in post-cystectomy patients are: the complexity of pelvic surgery, extensive lymphadenectomy, prolonged procedure, extended time in lithotomy position, and perhaps biochemical alterations in the coagulation cascade due to the malignancy itself. The comparison to other solid tumors associated with a lower incidence of VTE may also be biased, given that patients who were offered surgery may have been at a lower risk of VTE in general.
As shown in Table 1, the risk of VTE in patients undergoing treatment with systemic chemotherapy ranges from 3.3% to 21%.[30,34-37] Multiple studies have looked at the incidence of VTE in this setting, and have identified risk factors for its development. In a study of 271 BCa patients undergoing cispla-tin-based therapy, 35 (12.9%) experienced a "vascular event." There were 18 DVT events, 9 PEs, 7 cases of arterial thrombosis, 3 strokes, and one case of superficial thrombophlebitis. Risk factors included a pelvic mass, existing peripheral vascular disease, and coronary artery disease.[35] A lower-dose regimen of gemcitabine and cisplatin was compared to standard dosing in a phase II trial in 2014 that revealed a DVT/PE rate of 3.3% in both arms.[37] However, with new therapies being instituted, such as bevacizumab, the toxicity profile will change in patients undergoing systemic chemotherapy. In the phase II trial of gemcitabine, cisplatin, and bevacizumab, an impressive 21% of patients experienced a DVT or PE.[36]
The ACCP, ASCO, ESMO, and NCCN guidelines recommend VTE prophylaxis with low-molecular-weight heparin (LMWH) or low-dose heparin (LDH) for hospitalized patients with active BCa who are not at significant risk of bleeding.[38-41] For hospitalized patients at risk of bleeding, graduated compression stockings (GCS) or intermittent pneumatic compression (IPC) is recommended. Note that routine prophylaxis is not recommended for ambulatory patients with BCa who are receiving outpatient chemotherapy, even those who have had a central venous catheter placed.
For BCa patients undergoing surgery, VTE prophylaxis recommendations are a bit more complicated. For minor outpatient procedures like transurethral resection of bladder tumor (TURBT), early ambulation alone is recommended in the American Urology Association (AUA) and ACCP guidelines because the VTE risk is very low.[42,43] However, radical cystectomy poses a much higher risk of VTE, and the ACCP, ASCO, AUA, ESMO, and NCCN guidelines all recommend pharmacologic prophylaxis (with LMWH the favored agent) as the primary means of VTE prevention, with IPC or GCS as a secondary method.[38-40,42,43] Importantly, pharmacologic prophylaxis should be started prior to surgical incision and should continue for 4 weeks postoperatively.[39,40] This is because VTE occurs at a median of 14 days postoperatively from cystectomy, implying that at least 50% of VTE events will occur after discharge.[44] If an epidural catheter is used for postoperative pain control, it should be placed prior to preoperative administration of heparin, and LDH is generally preferred over LMWH (although LMWH is still an option) as long as the epidural catheter is in place, because LDH has a shorter duration of action and is associated with a lower rate of epidural hematoma.[45] Routine screening for VTE with Doppler ultrasonography in postoperative BCa patients is not recommended.[43]
Bleeding in Bladder Cancer
Hematuria is the characteristic sign that typically leads to detection of BCa.[46] Up to 85% of patients with newly diagnosed BCa have gross hematuria, and virtually all have microscopic hematuria.[47-49] While 15% to 20% of patients with gross hematuria will have BCa, the risk of BCa in patients with microscopic hematuria is only 1% to 5%.[46-48,50] BCa-induced hematuria can also present more acutely as profuse bladder hemorrhage. Such cases require admission for bladder irrigation (usually with a three-way catheter and saline, although bladder irrigation is sometimes achieved with hemostatic irrigation solutions), red blood cell (RBC) transfusion, and occasionally operative intervention. The most common operative procedures include cystoscopic clot evacuation and transurethral fulguration, with or without tumor resection. In rare cases of severe bleeding, intravesical formalin, bladder angioembolization, or urgent cystectomy may be indicated.[51]
A second scenario in which bleeding is encountered is during the surgical management of BCa. After TURBT, 1% to 2% of patients require a RBC transfusion and 0.5% to 2.5% require a second clot evacuation/hemostasis procedure.[52,53] Approximately 5% of patients are readmitted after TURBT, and bleeding requiring transfusion accounts for 3% of these readmissions.[54] Bipolar resection causes less blood loss than monopolar resection.[52]
Blood loss is a significantly bigger problem for patients managed with radical cystectomy, however. For open cystectomy, the median blood loss volume is 500-1,000 mL, the median rate of RBC transfusion is 40% to 70%, and the median number of transfused RBC units is 2.[4,55-58] The amount of blood loss in patients who undergo robotic/laparoscopic cystectomy is approximately half that of open cystectomy; consequently, the transfusion rate is quite a bit lower.[55,59] The transfusion rate is clearly affected by neoadjuvant chemotherapy, which is standard of care for muscle-invasive BCa, since these patients are often anemic preoperatively after chemotherapy. Several studies have shown a 15% to 30% increased risk of cancer-specific and overall mortality in patients who receive perioperative RBC transfusions at cystectomy.[4,56,58] While a variety of immune mechanisms have been proposed to explain this phenomenon, we believe that the relationship between transfusion and outcome is clearly confounded by disease severity, which may not be fully captured by the clinical variables currently considered in survival models.
Regardless of whether RBC transfusion worsens cancer outcomes, it is still desirable to reduce the number of transfusions performed, since they are expensive and increase the risk of adverse events such as allergic transfusion reactions, transfusion-associated lung injury, and infections.[60] Several approaches to reducing blood loss from cystectomy and/or the need for RBC transfusions are described in Table 2.
As mentioned previously, a number of biochemical changes in the coagulation and fibrinolytic cascades occur in the setting of BCa. Clinical evidence of these changes is of course the hematuria seen at presentation; however, systemic derangements can be seen as well. De novo development of DIC with microvascular thrombosis is a known entity in BCa. There have been multiple case reports of DIC occurring in patients with BCa, particularly after tumor manipulation (ie, surgical intervention). This phenomenon is also seen in other solid tumors, and several mechanisms have been proposed to explain how it may occur. TF is believed to play a role, as it is overexpressed in bladder cancer. Its interaction with factor VIIa triggers both prothrombinase and tenase complexes that will generate thrombin and lead to DIC with coagulation factor consumption. Another proposed mechanism is overexpression of urokinase, which may add to the fibrinolytic activity leading to increased bleeding. Another risk factor is the presence of necrosis within the tumor, which creates endothelial cell damage, triggering the intrinsic coagulation pathway within its microenvironment. The activation of this pathway may lead to aberrant shifts in hemostasis, such as development of DIC and consequent consumptive coagulopathy.[61-63]
Conclusions
In the treatment of BCa, it is essential that the clinician take into account the entire hemostatic spectrum, from bleeding to clotting. Hematuria is the hallmark of BCa, and is seen in nearly 100% of diagnosed cases. In reviewing the literature, it became clear that the proper clinical approach to patients with hematuria who are at risk for BCa is not taken as frequently as we would expect. Improper management introduces the potential for missed diagnoses and delays in treatment, which are especially problematic in BCa patients with high-grade disease. As a urologic community we must raise awareness about the clinical implications of hematuria, and ensure that the proper patient workup and referrals are being carried out.
Operative blood loss is unavoidable in both endoscopic and extirpative treatment of BCa. Urologists perform a wide array of outpatient procedures, and the literature reviewed in this article indicates that TURBT is the most common procedure in this setting. In turn, the most common reason for readmission following TURBT has been development of gross hematuria requiring bladder irrigation. This likely mirrors the practice patterns of many general urologists working in the community setting. Avoidance of this particular problem will vary from one institution to another, depending on whether it is a low-volume or a high-volume center, a teaching or non-teaching hospital, or a community-based private practice. However, adequate resection with aggressive intraoperative hemostatic control using bipolar cautery will be paramount in all of these settings.
Controlling blood loss in radical cystectomy will continue to be a subject of debate in the urologic literature, and one of the major talking points among proponents of robot-assisted surgery. In the reviewed publications, the transfusion rates were quite high for patients managed with open radical cystectomy. Given these rates, coupled with the potential risks of transfusion reactions and perhaps even a negative oncologic effect of allogeneic blood products, strategies for reducing blood loss are going to be continually sought after. It is our opinion that experienced surgeons working in high-volume institutions will be able to achieve the best clinical outcomes, due to the multidisciplinary ancillary support available at such centers, and the complex nature of the surgery. Adjunctive treatments, such as cell-salvage, blood banking, improved vascular pedicle management, and systemic clotting agents, may be of some clinical benefit in the perioperative period. Minimally invasive robotic surgery does appear to have impressive perioperative outcomes; however, the oncologic durability of this procedure is not entirely clear at this time. Given that the treatment goal is removal of the malignant process with subsequent cancer-free survival, this is the measure that should be used to determine the long-term success of the procedure. Further investigation into the use of intra-operative systemic hemostatic agents, such as desmopressin, tranexamic acid, factor 7, and aminocaproic acid, which have proven useful in other surgical contexts, is needed in cystectomy. However, the risk of undesirable thrombosis attributable to these pharmacologic agents must be taken into consideration along with the underlying propensity for clot formation in patients with BCa.
Reliable evidence of biochemical alterations in both the coagulation and fibrinolytic cascades has been reported in the setting of BCa. Some of these altered molecules are even the targets of treatments now under development, given their association with more aggressive disease. These alterations do seem to translate to clinical consequences in the management BCa, ranging from fulminant DIC to PE. The 6% rate of VTE post radical cystectomy, with 20% of VTEs being fatal despite appropriate prophylaxis,[64] shows us that aberrant clotting in this setting can have devastating clinical consequences. Early ambulation, institution of DVT chemoprophylaxis as soon as feasible, and awareness by the treatment team that VTE is a risk should be the cornerstones of VTE prevention and early recognition. VTE in the setting of systemic chemotherapy is also of great concern, and is likely due in part to both the disease process itself and the toxicity of the agent. Screening for DVT in the setting of advanced BCa is not of benefit at this time. There is a need for further work on the development of novel prophylaxis approaches, preoperative risk stratification, and perhaps preoperative interventions to reduce the incidence of postoperative clotting. All of these investigations must take into consideration the propensity of bleeding in this population and weigh it against their aberrant clotting risk.
Financial Disclosure: The authors have no significant financial interest or other relationship with the manufacturers of any products or providers of any service mentioned in this article.
References:
1. Sandhu R, Pan CX, Wun T, et al. The incidence of venous thromboembolism and its effect on survival among patients with primary bladder cancer. Cancer. 2010;116:2596-603.
2. Lyman GH. Venous thromboembolism in the patient with cancer: focus on burden of disease and benefits of thromboprophylaxis. Cancer. 2011;117:1334-49.
3. Chew HK, Wun T, Harvey D, et al. Incidence of venous thromboembolism and its effect on survival among patients with common cancers. Arch Intern Med. 2006;166:458-64.
4. Linder BJ, Frank I, Cheville JC, et al. The impact of perioperative blood transfusion on cancer recurrence and survival following radical cystectomy. Eur Urol. 2013;63:839-45.
5. Hisazumi H. Inhibitory effect of normal and cancerous tissues of the bladder on plasminogen activation. Invest Urol. 1973;11:258-62.
6. Hisazumi H, Naito K, Misaki T. Fibrinolytic activity in normal and cancerous tissues of the bladder. Invest Urol. 1973;11:28-34.
7. Ladehoff A. The fibrinolytic activity of tumors of the kidney and bladder. Acta Pathol Microbiol Scand. 1962;55:273-80.
8. Adamson AS, Francis JL, Roath OS, et al. Urinary tissue factor levels in transitional cell carcinoma of the bladder. J Urol. 1992;148:449-52.
9. Lwaleed BA, Francis JL, Chisholm M. Urinary tissue factor levels in patients with bladder and prostate cancer. Eur J Surg Oncol-. 2000;26:44-9.
10. Förster Y, Meye A, Albrecht S, et al. Tissue specific expression and serum levels of human tissue factor in patients with urological cancer. Cancer Lett-. 2003;193:65-73.
11. Patry G, Hovington H, Larue H, et al. Tissue factor expression correlates with disease-specific survival in patients with node-negative muscle-invasive bladder cancer. Int J Cancer. 2008;122:1592-7.
12. Hudson MA, McReynolds LM. Urokinase and the urokinase receptor: association with in vitro invasiveness of human bladder cancer cell lines. J Natl Cancer Inst. 1997;89:709-17.
13. Casella R, Shariat SF, Monoski MA, Lerner SP. Urinary levels of urokinase-type plasminogen activator and its receptor in the detection of bladder carcinoma. Cancer. 2002;95:2494-9.
14. Hanke M, Kausch I, Dahmen G, et al. Detailed technical analysis of urine RNA-based tumor diagnostics reveals ETS2/urokinase plasminogen activator to be a novel marker for bladder cancer. Clin Chem. 2007;53:2070-7.
15. Hasui Y, Marutsuka K, Asada Y, Osada Y. Prognostic value of urokinase-type plasminogen activator in patients with superficial bladder cancer. Urology. 1996;47:34-7.
16. Shariat SF, Monoski MA, Andrews B, et al. Association of plasma urokinase-type plasminogen activator and its receptor with clinical outcome in patients undergoing radical cystectomy for transitional cell carcinoma of the bladder. Urology. 2003;61:1053-8.
17. Hisazumi H, Uchibayashi T, Naito K, et al. The prophylactic use of thiotepa and urokinase in transitional cell carcinoma of the bladder: a preliminary report. J Urol. 1975;114:394-8.
18. Khan O, Aherne GW, Williams G. Combined intravesical therapy with doxorubicin (adriamycin) and urokinase in the management of superficial bladder tumours. Br J Urol. 1982;54:280-2.
19. Lundbeck F, Mogensen P, Jeppesen N. Intravesical therapy of noninvasive bladder tumors (stage Ta) with doxorubicin and urokinase. J Urol. 1983;130:1087-9.
20. Ulisse S, Baldini E, Sorrenti S, D̢۪Armiento M. The urokinase plasminogen activator system: a target for anti-cancer therapy. Curr Cancer Drug Targets. 2009;9:32-71.
21. Chen LM, Chang M, Dai Y, et al. External validation of a multiplex urinary protein panel for the detection of bladder cancer in a multicenter cohort. Cancer Epidemiol Biomarkers Prev. 2014;23:1804-12.
22. Becker M, Szarvas T, Wittschier M, et al. Prognostic impact of plasminogen activator inhibitor type 1 expression in bladder cancer. Cancer. 2010;116:4502-12.
23. Gomes-Giacoia E, Miyake M, Goodison S, Rosser CJ. Targeting plasminogen activator inhibitor-1 inhibits angiogenesis and tumor growth in a human cancer xenograft model. Mol Cancer Ther. 2013;12:2697-708.
24. Topsakal M, Karadeniz T, Anac M, et al. Assessment of fibrin-fibrinogen degradation products (Accu-Dx) test in bladder cancer patients. Eur Urol. 2001;39:287-91.
25. Schmetter BS, Habicht KK, Lamm DL, et al. A multicenter trial evaluation of the fibrin/fibrinogen degradation products test for detection and monitoring of bladder cancer. J Urol. 1997;158:801-5.
26. Martinez-Pineiro JA, Pertusa C, Maganto E, et al. Urinary fibrinogen degradation products (FDP) in bladder cancer. Eur Urol. 1978;4:348-50.
27. Mizutani Y, Nio Y, Fukumoto M, Yoshida O. Enhanced antitumor effect of Bacillus Calmette-Guerin in combination with fibrinogen on urinary bladder tumor. J Urol. 1994;151:1420-6.
28. Khorana AA, McCrae KR. Risk stratification strategies for cancer-associated thrombosis: an update. Thromb Res. 2014;133(Suppl 2):S35-8.
29. Caprini JA. Risk assessment as a guide for the prevention of the many faces of venous thromboembolism. Am J Surg. 2010;199:S3-10.
30. Khorana AA, Dalal M, Lin J, Connolly GC. Incidence and predictors of venous thromboembolism (VTE) among ambulatory high-risk cancer patients undergoing chemotherapy in the United States. Cancer. 2013;119:648-55.
31. Chew HK, Wun T, Harvey DJ, et al. Incidence of venous thromboembolism and the impact on survival in breast cancer patients. J Clin Oncol. 2007;25:70-6.
32. Chew HK, Davies AM, Wun T, et al. The incidence of venous thromboembolism among patients with primary lung cancer. J Thromb Haemost. 2008;6:601-8.
33. Alcalay A, Wun T, Khatri V, et al. Venous thromboembolism in patients with colorectal cancer: incidence and effect on survival. J Clin Oncol. 2006;24:1112-8.
34. Agnelli G, George DJ, Kakkar AK, et al. Semuloparin for thromboprophylaxis in patients receiving chemotherapy for cancer. N Engl J Med. 2012;366:601-9.
35. Czaykowski PM, Moore MJ, Tannock IF. High risk of vascular events in patients with urothelial transitional cell carcinoma treated with cisplatin based chemotherapy. J Urol. 1998;160:2021-4.
36. Hahn NM, Stadler WM, Zon RT, et al. Phase II trial of cisplatin, gemcitabine, and bevacizumab as first-line therapy for metastatic urothelial carcinoma: Hoosier Oncology Group GU 04-75. J Clin Oncol. 2011;29:1525-30.
37. Haggag R, Farag K, Abu-Taleb F, et al. Low-dose versus standard-dose gemcitabine infusion and cisplatin for patients with advanced bladder cancer: a randomized phase II trial—an update. Med Oncol. 2014;31:811.
38. Streiff MB, Bockenstedt PL, Cataland SR, et al. Venous thromboembolic disease. J Natl Compr Canc Netw. 2013;11:1402-29.
39. Mandala M, Falanga A, Roila F. Management of venous thromboembolism (VTE) in cancer patients: ESMO clinical practice guidelines. Ann Oncol. 2011;22 (suppl 6):vi85-92.
40. Lyman GH, Khorana AA, Kuderer NM, et al. Venous thromboembolism prophylaxis and treatment in patients with cancer: American Society of Clinical Oncology clinical practice guideline update. J Clin Oncol. 2013;31:2189-204.
41. Kahn SR, Lim W, Dunn AS, et al. Prevention of VTE in nonsurgical patients: antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2012;141:e195S-226S.
42. Forrest JB, Clemens JQ, Finamore P, et al. AUA best practice statement for the prevention of deep vein thrombosis in patients undergoing urologic surgery. J Urol. 2009;181:1170-7.
43. Gould MK, Garcia DA, Wren SM, et al. Prevention of VTE in nonorthopedic surgical patients: antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2012;141:e227S-77S.
44. VanDlac AA, Cowan NG, Chen Y, et al. Timing, incidence and risk factors for venous thromboembolism in patients undergoing radical cystectomy for malignancy: a case for extended duration pharmacological prophylaxis. J Urol. 2014;191:943-7.
45. Horlocker TT, Wedel DJ, Rowlingson JC, et al. Regional anesthesia in the patient receiving antithrombotic or thrombolytic therapy: American Society of Regional Anesthesia and Pain Medicine evidence-based guidelines (3rd ed). Reg Anesth Pain Med. 2010;35:64-101.
46. Davis R, Jones JS, Barocas DA, et al. Diagnosis, evaluation and follow-up of asymptomatic microhematuria (AMH) in adults: AUA guideline. J Urol. 2012;188:2473-81.
47. Khadra MH, Pickard RS, Charlton M, et al. A prospective analysis of 1,930 patients with hematuria to evaluate current diagnostic practice. J Urol. 2000;163:524-7.
48. Edwards TJ, Dickinson AJ, Natale S, et al. A prospective analysis of the diagnostic yield resulting from the attendance of 4020 patients at a protocol-driven haematuria clinic. BJU Int. 2006;97:301-5.
49. Alishahi S, Byrne D, Goodman CM, Baxby K. Haematuria investigation based on a standard protocol: emphasis on the diagnosis of urological malignancy. J R Coll Surg Edinb. 2002;47:422-7.
50. Mishriki SF, Grimsley SJ, Nabi G. Incidence of recurrent frank hematuria and urological cancers: prospective 6.9 years of followup. J Urol. 2009;182:1294-8.
51. Abt D, Bywater M, Engeler DS, Schmid HP. Therapeutic options for intractable hematuria in advanced bladder cancer. Int J Urol. 2013;20:651-60.
52. Sugihara T, Yasunaga H, Horiguchi H, et al. Comparison of perioperative outcomes including severe bladder injury between monopolar and bipolar transurethral resection of bladder tumors: a population-based comparison. J Urol. 2014 Jun 2. [Epub ahead of print]
53. Collado A, Chechile GE, Salvador J, Vicente J. Early complications of endoscopic treatment for superficial bladder tumors. J Urol. 2000;164:1529-32.
54. Rambachan A, Matulewicz RS, Pilecki M, et al. Predictors of readmission following outpatient urological surgery. J Urol. 2014 Feb 8. [Epub ahead of print]
55. Tang K, Li H, Xia D, et al. Laparoscopic versus open radical cystectomy in bladder cancer: a systematic review and meta-analysis of comparative studies. PLOS ONE. 2014;9:e95667.
56. Morgan TM, Barocas DA, Chang SS, et al. The relationship between perioperative blood transfusion and overall mortality in patients undergoing radical cystectomy for bladder cancer. Urol Oncol. 2013;31:871-7.
57. Thompson IM 3rd, Kappa SF, Morgan TM, et al. Blood loss associated with radical cystectomy: a prospective, randomized study comparing Impact LigaSure vs. stapling device. Urol Oncol. 2014;32:45.e11-5.
58. Kluth LA, Xylinas E, Rieken M, et al. Impact of peri-operative blood transfusion on the outcomes of patients undergoing radical cystectomy for urothelial carcinoma of the bladder. BJU Int. 2014;113:393-8.
59. Bochner BH, Sjoberg DD, Laudone VP. A randomized trial of robot-assisted laparoscopic radical cystectomy. N Engl J Med. 2014;371:389-90.
60. Practice guidelines for perioperative blood transfusion and adjuvant therapies: an updated report by the American Society of Anesthesiologists Task Force on Perioperative Blood Transfusion and Adjuvant Therapies. Anesthesiology. 2006;105:198-208.
61. Colman RW, Rubin RN. Disseminated intravascular coagulation due to malignancy. Semin Oncol. 1990;17:172-86.
62. Sallah S, Wan JY, Nguyen NP, et al. Disseminated intravascular coagulation in solid tumors: clinical and pathologic study. Thromb Haemost. 2001;86:828-33.
63. Tauzin-Fin P, Sesay M, Ryman A, et al. Postoperative thrombotic microangiopathy following radical cystectomy for bladder cancer. Anaesth Intensive Care. 2006;34:672-5.
64. Blom JW, Vanderschoot JP, Oostindier MJ, et al. Incidence of venous thrombosis in a large cohort of 66,329 cancer patients: results of a record linkage study. J Thromb Haemost. 2006;4:529-35.
65. Clement C, Rossi P, Aissi K, et al. Incidence, risk profile and morphological pattern of lower extremity venous thromboembolism after urological cancer surgery. J Urol. 2011;186:2293-7.
66. Dyer J, Wyke S, Lynch C. Hospital Episode Statistics data analysis of postoperative venous thromboembolus in patients undergoing urological surgery: a review of 126,891 cases. Ann R Coll Surg Engl. 2013;95:65-9.
67. Albers P, Heicappell R, Schwaibold H, Wolff J. Erythropoietin in urologic oncology. Eur Urol. 2001;39:1-8.
68. Case DC Jr, Bukowski RM, Carey RW, et al. Recombinant human erythropoietin therapy for anemic cancer patients on combination chemotherapy. J Natl Cancer Inst. 1993;85:801-6.
69. Dunphy FR, Harrison BR, Dunleavy TL, et al. Erythropoietin reduces anemia and transfusions: a randomized trial with or without erythropoietin during chemotherapy. Cancer. 1999;86:1362-7.
70. Maegawa M, Usida H, Maekawa S, et al. [The usefulness of autologous blood transfusion for urologic surgery.] Hinyokika Kiyo. 2001;47:625-8.
71. Hayakawa K, Sato H, Aoyagi T, et al. [The use of predeposited autologous blood transfusion for radical prostatectomy and total cystectomy.] Nihon Hinyokika Gakkai Zasshi. 1999;90:496-501.
72. Chang SS, Smith JA Jr, Cookson MS. Decreasing blood loss in patients treated with radical cystectomy: a prospective randomized trial using a new stapling device. J Urol. 2003;169:951-4.
73. Ubee SS, Manikandan R, Gudimetla AR, Singh G. Cost benefits of intraoperative cell salvage in radical cystectomy. Indian J Urol. 2010;26:196-9.
74. Nieder AM, Manoharan M, Yang Y, Soloway MS. Intraoperative cell salvage during radical cystectomy does not affect long-term survival. Urology. 2007;69:881-4.
75. Aning J, Dunn J, Daugherty M, et al. Towards bloodless cystectomy: a 10-year experience of intra-operative cell salvage during radical cystectomy. BJU Int. 2012;110:E608-13.
76. Ashworth A, Klein AA. Cell salvage as part of a blood conservation strategy in anaesthesia. Br J Anaesth. 2010;105:401-16.
77. Davies L, Brown TJ, Haynes S, et al. Cost-effectiveness of cell salvage and alternative methods of minimising perioperative allogeneic blood transfusion: a systematic review and economic model. Health Technol Assess. 2006;10:iii-iv, ix-x, 1-210.
78. Punjani N, Lavallee LT, Momoli F, et al. Blood transfusion and hemostatic agents used during radical cystectomy. Can Urol Assoc J. 2013;7:E275-80.
79. Seyednejad H, Imani M, Jamieson T, Seifalian AM. Topical haemostatic agents. Br J Surg. 2008;95:1197-225.
80. Ahlering TE, Henderson JB, Skinner DG. Controlled hypotensive anesthesia to reduce blood loss in radical cystectomy for bladder cancer. J Urol. 1983;129:953-4.
81. Ryan DW. Anaesthesia for cystectomy. A comparison of two anaesthetic techniques. Anaesthesia. 1982;37:554-60.
82. Gille J, Winter V, Sablotzki A, et al. Presurgical hyper-volaemic haemodilution for saving blood transfusion? Eur J Anaesthesiol. 2008;25:172-3.
83. Takayanagi A, Masumori N, Kobayashi K, et al. Acute normovolemic hemodilution for radical retropubic prostatectomy and radical cystectomy. Urology. 2008;72:401-5.
84. Fenger-Eriksen C, Jensen TM, Kristensen BS, et al. Fibrinogen substitution improves whole blood clot firmness after dilution with hydroxyethyl starch in bleeding patients undergoing radical cystectomy: a randomized, placebo-controlled clinical trial. J Thromb Haemost. 2009;7:795-802.
85. Breau RH, Kokolo MB, Punjani N, et al. The effects of lysine analogs during pelvic surgery: a systematic review and meta-analysis. Transfus Med Rev. 2014; 28:145-55.