New Advances in Ovarian Cancer
Epithelial ovarian cancer is the leading cause of death from gynecologic malignancy in the United States, with approximately 15,000 deaths per year. Platinum/taxane doublets have long been considered the standard treatment regimen for advanced-stage disease; however, recent studies have sought to improve on the outcome from this therapy. Intraperitoneal (IP) chemotherapy has been shown to yield superior progression-free survival (PFS) and overall survival (OS); however, logistical problems and toxicities have limited more widespread adoption. Recent studies have also suggested that a “dose-dense” schedule of paclitaxel in combination with carboplatin may result in improved outcomes, and the impact of biological therapies in the first-line setting is under active investigation. In the setting of recurrent disease, preliminary results suggest that novel doublet regimens such as carboplatin and pegylated liposomal doxorubicin may have similar activity to standard platinum/taxane doublets while carrying a reduced risk of allergic reactions. Additionally, targeted therapy remains an active area of investigation, with evidence of activity from agents such as PARP inhibitors, anti-angiogenics, and PI3 kinase inhibitors. Here, we review recent advances in our understanding of ovarian cancer and its treatment in both the newly diagnosed and recurrent settings.
Epithelial ovarian cancer is the leading cause of death from gynecologic malignancy in the United States, with approximately 15,000 deaths per year. Platinum/taxane doublets have long been considered the standard treatment regimen for advanced-stage disease; however, recent studies have sought to improve on the outcome from this therapy. Intraperitoneal (IP) chemotherapy has been shown to yield superior progression-free survival (PFS) and overall survival (OS); however, logistical problems and toxicities have limited more widespread adoption. Recent studies have also suggested that a “dose-dense” schedule of paclitaxel in combination with carboplatin may result in improved outcomes, and the impact of biological therapies in the first-line setting is under active investigation. In the setting of recurrent disease, preliminary results suggest that novel doublet regimens such as carboplatin and pegylated liposomal doxorubicin may have similar activity to standard platinum/taxane doublets while carrying a reduced risk of allergic reactions. Additionally, targeted therapy remains an active area of investigation, with evidence of activity from agents such as PARP inhibitors, anti-angiogenics, and PI3 kinase inhibitors. Here, we review recent advances in our understanding of ovarian cancer and its treatment in both the newly diagnosed and recurrent settings.
TABLE 1

International Federation of Gynecology and Obstetrics (FIGO) Staging for Epithelial Ovarian Cancer
Epithelial ovarian cancer is diagnosed in an estimated 21,550 women in the United States per year and was responsible for 14,600 deaths in 2009; this cancer remains the leading cause of death from gynecologic malignancy in the United States.[1] The vast majority of cases are diagnosed at a late stage (FIGO stage III or IV); FIGO staging for ovarian cancer is as per Table 1,[2] and initial cytoreductive surgery is recommended for patients who are surgical candidates, followed by adjuvant chemotherapy.[3] Ovarian cancer is distinguished by initial chemotherapy sensitivity; the majority of ovarian cancers will demonstrate a high response rate (~75%-80%) to first-line platinum- and taxane-based chemotherapy.[4] However, most patients subsequently have disease relapse within 12 to 18 months.[5] Recent studies have introduced new perspectives on the potential cell of origin of presumed high-grade serous ovarian cancer, as well as new approaches towards management of both newly diagnosed and recurrent disease. In this article, we will review recent advances in our understanding of the pathogenesis and management of epithelial ovarian cancer.
The Distal Fallopian Tube as Site of Origin
The pathogenesis of epithelial ovarian carcinomas is varied depending on histology. Type 1 ovarian cancers encompass low-grade serous and endometrioid as well as mucinous subtypes, and often demonstrate mutations in KRAS, BRAF, or PTEN.[6] In contrast, the majority of epithelial ovarian cancers are high-grade serous carcinomas that are frequently associated with mutations in TP53.[6] A long-standing model of ovarian cancer pathogenesis has suggested that the repeated trauma and repair associated with ovulation contributes to the development of ovarian carcinoma from ovarian surface epithelial cells.[7] However, the observation that there is a significantly increased percentage of fallopian tube lesions in high-risk patients undergoing prophylactic bilateral salpingo-oophorectomy has raised the question of whether a portion of serous pelvic tumors may originate from the fimbria of the fallopian tube.[6] This distribution of fallopian tube lesions has prompted additional investigation.[8] In one study, a "p53 signature," where small segments of normal-appearing fallopian tube epithelium demonstrated strong p53 immunohistochemistry, was identified; this was often associated with mutation of the TP53 gene.[8] Additionally, TP53 mutations were detected in all of the tubal intraepithelial carcinomas (TICs) tested, and in one case, the same mutation was shared by a contiguous "p53 signature" and TIC. These results suggest that the fallopian tube should be considered as a possible field of origin for pelvic serous carcinomas, and investigation currently continues in this area.
Treatment for Newly Diagnosed Ovarian Cancer
Neoadjuvant Chemotherapy and Timing of Cytoreductive Surgery
The approach to treatment for newly diagnosed ovarian, fallopian tube, and primary peritoneal cancers has typically been the same. Since Griffiths demonstrated the presence of an inverse relationship between overall survival and residual size,[9] aggressive cytoreductive surgery has played an important role in the initial management of these cancers, and current NCCN guidelines suggest that cytoreductive surgery be considered in the initial treatment of newly diagnosed ovarian cancer.[3] However, recent results from the European Organisation for Research and Treatment of Cancer–Gynecologic Cancer Group (EORTC–GCG) and the National Cancer Institute of Canada Clinical Trials Group (NCIC-CTG) examining the efficacy and outcomes of primary neoadjuvant chemotherapy has raised the question of when patients should ideally undergo cytoreductive surgery.[10] In this study, 718 patients with stage IIIC or IV ovarian cancer were randomized to receive either primary debulking surgery followed by 6 cycles of IV platinum-based chemotherapy or 3 cycles of neoadjuvant IV chemotherapy, followed by interval debulking surgery and then an additional 3 cycles of IV chemotherapy. Optimal cytoreduction (defined as residual ≤1 cm) was achieved in 48% of patients receiving primary debulking surgery and 83% of patients who received neoadjuvant chemotherapy. Additionally, patients who underwent primary debulking surgery had higher rates of complications, including post-operative mortality (2.7% vs 0.6%), sepsis (8% vs 2%), and grade 3 or 4 hemorrhage (7% vs 4%). The study, which was powered for non-inferiority, demonstrated no difference in median OS (29 vs 30 months) or PFS (11 months in both groups). Based upon these results, the authors concluded that neoadjuvant chemotherapy should be considered, given the similar survival outcomes and the increased relative morbidity of primary debulking surgery. Of note, this study did not require that surgery be performed by gynecologic oncologists, which in prior studies has been shown to result in higher rates of optimal cytoreduction and improved survival outcomes.[11] The final peer-reviewed publication of these results has not yet occurred, and there have been no changes to recommendations with regard to the timing of primary debulking surgery.[3]
Intravenous (IV) Chemotherapy
Despite recent studies with IP chemotherapy (discussed later in this article), IV chemotherapy is commonly used in patients with newly diagnosed ovarian cancer and is the treatment of choice for patients with suboptimally cytoreduced (residual cancer >1 cm) disease, poor performance status, contraindications to IP chemotherapy, and/or who decline IP treatment. The standard IV regimen of platinum and paclitaxel was established in a study by McGuire et al. in 1996 in which 410 patients with suboptimally debulked stage III or IV disease were randomized to receive either IV cisplatin and cyclophosphamide or IV cisplatin and paclitaxel.[4] Response rates were significantly higher in the paclitaxel-containing arm (73% vs 60%, P = .01), and OS was also significantly longer (38 months vs 24 months, P < .001). As subsequent trials have demonstrated that carboplatin can result in equal efficacy but less toxicity than cisplatin in this setting, [5] a carboplatin-based regimen (including paclitaxel) has been considered a standard of care for IV chemotherapy in advanced ovarian cancer.[3]
Although the dosing of IV carboplatin/paclitaxel has typically been administered once every 21 days, a recently reported trial from Japan has suggested that a dose-dense regimen of weekly paclitaxel in combination with carboplatin given once every 3 weeks may improve outcomes.[12] In this open-label phase III study, 637 patients were randomized to receive 6 cycles of either paclitaxel 180 mg/m2 and carboplatin AUC 6 dosed once every 21 days or "dose-dense" paclitaxel at 80 mg/m2 on days 1, 8, and 15 and carboplatin AUC 6 on day 1 of a 21-day cycle. Median PFS was longer in the dose-dense group (28.0 months vs 17.2 months, P = .0015), and OS at 3 years was also statistically superior in the dose-dense group (72.1% vs 65.1%, P = .03). Grade 3 or 4 anemia was more common in the dose-dense group, but other toxicities were similar between the two treatment arms. These results have raised the question of whether a dose-dense schedule should be considered for patients receiving first-line IV chemotherapy and could represent a new option for the treatment of appropriate patients with advanced ovarian, peritoneal, or fallopian tube cancer.
Other studies have sought to improve the outcomes observed with IV carboplatin and paclitaxel by adding additional drugs to a platinum-doublet backbone. Bookman et al conducted a 4,312-patient study of women with newly diagnosed, advanced ovarian cancer that encompassed 5 arms: 1) a control arm of IV carboplatin AUC 6 and paclitaxel 175 mg/m2 every 21 days × 8 cycles; 2) carboplatin AUC 5 and paclitaxel 175 mg/m2 on day 1 and gemcitabine 800 mg/m2 on days 1 and 8, every 21 days × 8 cycles; 3) carboplatin AUC 5 and paclitaxel 175 mg/m2 every 21 days × 8 cycles and pegylated liposomal doxorubicin 30 mg/m2 on day 1 during cycles 1, 3, 5, and 7; 4) carboplatin AUC 5 on day 3 and topotecan 1.25 mg/m2/d on days 1, 2, 3, every 21 days × 4 cycles, followed by carboplatin AUC 6 and paclitaxel 175 mg/m2 every 21 days × 4 cycles; and 5) carboplatin AUC 6 on day 8 and gemcitabine 1,000 mg/m2 on days 1 and 8, every 21 days × 4 cycles, followed by carboplatin AUC 6 and paclitaxel 175 mg/m2 × 4 cycles.[13] There were no differences observed among the five arms with respect to PFS and OS; thus, additional drugs added to platinum/taxane or the use of different platinum doublets did not demonstrate any improvement in clinical outcome.
Similarly, results from the Gynecologic Cancer Intergroup (GCIG) study AGO-OVAR-9, which compared a regimen of 6 cycles of carboplatin, platinum, and gemcitabine against carboplatin and paclitaxel alone have also been presented, and no benefit was observed from addition of gemcitabine to the control regimen.[14] More recently, preliminary results were presented from a phase III study comparing the combination of carboplatin and pegylated liposomal doxorubicin in the first-line setting from the Mulitcentre Italian Trials in Ovarian Cancer (MITO-2) study.[15] Response rates to these regimens appeared to be similar between the two treatment arms, and the final PFS analysis is pending. Given the results of these studies, carboplatin/paclitaxel remains the standard of care doublet for newly diagnosed disease.[3]
Biological Therapies
TABLE 2
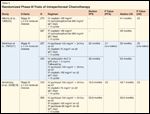
Randomized Studies Demonstrating Benefit of Combination Versus Single-Agent Platinum-Based Therapy in Recurrent Platinum-Sensitive Ovarian Cancer
The role of biologic therapies in the first-line treatment of newly diagnosed ovarian cancer is under active exploration. Anti-angiogenic agents have demonstrated activity in the recurrent setting, and two trials are currently investigating whether the addition of bevacizumab to first-line treatment will improve clinical outcomes. The Gynecologic Oncology Group (GOG) has completed enrollment of a large randomized placebo-controlled phase III trial (GOG 218) investigating the benefit of concurrent and maintenance bevacizumab with carboplatin and paclitaxel in newly diagnosed ovarian cancer, and preliminary results from this trial were presented at the ASCO meeting in June 2010.[16] Three arms are included in this trial: 1) IV carboplatin, paclitaxel, and placebo for 6 cycles, followed by maintenance placebo for 1 year; 2) IV carboplatin, paclitaxel, and bevacizumab for 6 cycles, followed by maintenance placebo for 1 year; and 3) IV carboplatin, paclitaxel, and bevacizumab for 6 cycles, followed by maintenance bevacizumab for 1 year. The primary endpoint for this study was PFS, with a statistical plan to analyze each of the bevacizumab-containing arms individually against the control arm. If both of the bevacizumab-containing arms demonstrated benefit compared to the control, the bevacizumab arms would be compared to one another. A total of 1,873 patients was accrued to this trial, with a median follow-up of 17.4 months. A significant difference in PFS of 14.1 months versus 10.3 months (P < .0001) was observed favoring Arm 3 (carboplatin/taxol/bevacizumab followed by 1 year of maintenance bevacizumab) as compared to the control arm (carboplatin/taxol alone). No difference was observed in PFS between Arm 2 (carboplatin/taxol/bevacizumab followed by 1 year of maintenance placebo) and the control arm. The study was not powered to observe differences in OS, but preliminary data collected at the time of final PFS analysis did not reveal any differences between the three arms (39.3 months for Arm 1 vs 38.7 months for Arm 2 vs 39.7 months for Arm 3). A second randomized open-label phase III trial (ICON7) comparing IV carboplatin and paclitaxel to a regimen of IV carboplatin, paclitaxel, and bevacizumab followed by bevacizumab maintenance in the same setting has also completed enrollment.[17] Additional biological therapies are also being explored in combination with chemotherapy in the first-line setting, and enrollment has recently begun for a phase III study (AGO-OVAR-12) with the oral anti-angiogenic agent BIBF 1120 in combination with carboplatin and paclitaxel in patients with advanced epithelial ovarian cancer.[18]
Intraperitoneal (IP) chemotherapy
The role and dosing of IP chemotherapy in newly diagnosed optimally debulked ovarian cancer remains an area of discussion. The most recent randomized phase III study, published in January 2006, compared a regimen of paclitaxel 135 mg/m2 IV over 24 hours day 1 and IV cisplatin 75 mg/m2 day 2 to a regimen of IV paclitaxel 135 mg/m2 IV over 24 hours day 1, IP cisplatin 100 mg/m2 day 2, and IP paclitaxel 60 mg/m2 day 8, and demonstrated significant benefits in PFS and OS, with an OS of 65.6 months in the IP/IV arm compared to 49.7 months in the IV arm (P = .03).[19] These results were consistent with the findings of two prior randomized trials conducted in the United States that suggested survival benefits of IP therapy over IV therapy (summarized in Table 2)[20,21] and, based upon these, a clinical alert was issued by the National Cancer Institute (NCI) stating that IP chemotherapy should be considered and discussed with women who have newly diagnosed, optimally debulked, stage III disease.[22]
FIGURE 1
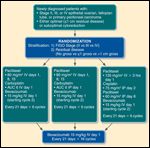
Trial Design Schema for GOG 252
Despite the above findings, adoption of IP chemotherapy as the standard of care for optimally cytoreduced stage III disease has been controversial. Logistical concerns regarding IP chemotherapy, including the 24-hour administration schedule for day 1 paclitaxel as well as the regimen's toxicities, have prevented more widespread adoption of IP chemotherapy.[23] Additionally, some centers have modified the GOG 172 regimen to reduce toxicities and decrease technical difficulties. Given the equivalent efficacy and reduced toxicity of IV carboplatin in comparison with IV cisplatin, the feasibility and efficacy of IP carboplatin has also been investigated.[24,25]
To further address the effectiveness of IP chemotherapy against current standard regimens, as well as to assess the potential of IP carboplatin chemotherapy, the GOG is now enrolling a phase III trial comparing a modified IP/IV GOG 172 cisplatin/paclitaxel regimen with an IP carboplatin/weekly paclitaxel and with an IV carboplatin/weekly paclitaxel regimen. In addition, bevacizumab will be added to the chemotherapy regimen on cycle 2 of each arm, and all three arms will also entail a year of bevacizumab maintenance therapy. A schema for this study (GOG 252) can be seen in Figure 1.
Monitoring
In the United States, recommendations for post-treatment monitoring following completion of first-line therapy typically include physical examination and CA-125 monitoring every 2 to 4 months for the first 2 years, with increasing intervals between follow-up visits if there is no evidence of disease recurrence.[3] However, data presented from the MRC OV05/EORTC 55955 study assessing the outcomes of early treatment at the time of biochemical recurrence versus delaying treatment until clinically indicated have suggested that early treatment does not alter overall survival.[26] In this study, 1,442 women with ovarian cancer in complete remission following first-line platinum-based therapy were followed every 3 months by CA-125, but both patients and physicians were blinded to the results. At the time a subject's CA-125 rose to twice the upper limit of normal, she was randomized to either initiation of treatment or continued blinded monitoring until treatment was indicated by other clinical parameters. Of note, choice of treatment was not dictated by the study design and was at the physician's discretion; the availability of clinical trials was also not specified. Although it remains unclear how these results will affect clinical practice, these findings do suggest that treatment of asymptomatic rises in CA-125 in the absence of recurrent cancer either on physical exam and/or radiographic study may not be warranted, and that these patients should be either closely observed or enrolled in clinical trials, if available.
Recurrent Disease
The majority of patients who have advanced-stage ovarian cancer at the time of initial diagnosis will experience recurrence despite the excellent response rate observed with first-line adjuvant chemotherapy. The choice of therapy for recurrent disease is often informed by the treatment-free interval (TFI) prior to disease recurrence, and recurrence is divided into platinum-sensitive (recurring greater than 6 months following treatment with a platinum-based regimen) or platinum-resistant (recurring within 6 months following prior platinum-based therapy). The TFI can predict response to additional therapy, and studies have demonstrated that patients with a longer platinum-free interval are more likely to respond to second-line platinum therapy.[27] Given these findings, the NCCN recommends consideration of a combination platinum-based chemotherapy in patients who have disease relapse greater than 6 months following their last platinum-based therapy.[3]
Combination Chemotherapies for Recurrent Disease
TABLE 3

Randomized Phase III Trials of Intraperitoneal Chemotherapy
Development of therapeutic treatments for recurrent platinum-sensitive disease has focused on combination regimens, based upon the findings from four randomized phase III trials and one phase II trial, which demonstrated a benefit to combination platinum-based chemotherapy compared to single-agent therapy (see Table 3).[28-31] Earlier trials investigated the combination of carboplatin and paclitaxel[28,29]; more recently, Pfisterer et al. demonstrated that carboplatin and gemcitabine also had increased activity compared to carboplatin alone in this setting.[30] The results of this trial led to the approval of the combination of carboplatin and gemcitabine for treatment of recurrent platinum-sensitive ovarian cancer in the United States.
Additional new doublets have been under exploration in platinum-sensitive disease. Preliminary results from the CALYPSO trial were presented at the American Society of Clinical Oncology (ASCO) meeting in June 2009.[32] In this phase III study, 976 patients with recurrent platinum-sensitive disease were randomized to receive either carboplatin and pegylated liposomal doxorubicin (PLD) or carboplatin and paclitaxel. PFS was significantly prolonged in the carboplatin/PLD arm (11.3 months vs 9.4 months). Of additional interest, the carboplatin/PLD arm experienced only a 5% hypersensitivity rate, compared to 18% in the carboplatin/pacltiaxel arm. The final results for this trial are not yet available, but these preliminary findings suggest a possible role for carboplatin/PLD in the treatment of recurrent platinum-sensitive ovarian cancer.
Non-platinum therapies also remain under investigation. Data were presented from a randomized phase III trial comparing the novel alkaloid trabectedin in combination with PLD to PLD alone.[33] A total of 672 patients were enrolled, and response rates for all patients were 28% in the combination arm compared to 19% in the PLD alone arm. There was no observed difference in overall survival; however, based upon the results of this trial, trabectedin has been approved in Europe for use in ovarian cancer, although the US FDA did not support approval of trabectedin in the United States.
Biologic/Targeted Therapies
Targeted biologic therapies are also a major area of interest in ovarian cancer research. Activity with the anti-angiogenic agent bevacizumab has been observed in phase II trials, with response rates of up to 16% in previously treated platinum-resistant recurrent ovarian cancer.[34,35] However, complications have also been observed with bevacizumab therapy in heavily pretreated populations, with one study reporting a gastrointestinal perforation rate of 11%.[35] Patients at higher risk included those who had received three or more prior chemotherapy regimens (including the initial chemotherapy regimen used at diagnosis); the risks of bevacizumab should be carefully considered for these patients as well as those who have extensive bowel involvement or symptoms of a partial or full small bowel obstruction. The activity of cediranib, an oral VEGF kinase inhibitor, has also been demonstrated in phase II trials in recurrent ovarian cancer.[36] A large randomized trial (ICON6) is investigating the utility of combining cediranib with carboplatin and paclitaxel therapy in women with platinum-sensitive relapsed ovarian cancer, and 450 patients are anticipated to be accrued to this study. Three arms are being studied in this trial: 1) standard carboplatin and paclitaxel with placebo therapy followed by maintenance placebo for 18 months, 2) carboplatin and paclitaxel with cediranib, followed by maintenance placebo for 18 months, and 3) carboplatin and paclitaxel with cediranib, followed by maintenance cediranib for 18 months.
One of the most exciting recent advances in targeted therapy in ovarian cancer has been the discovery and use of PARP-inhibitors. These agents work synergistically with the deficiencies of DNA-repair seen in cancers occurring in BRCA1- or BRCA2-carrier patients to damage DNA in tumor cells and cause tumor cell death.[37] In a phase I trial of an oral PARP-inhibitor, olaparib, 12 of 19 patients who were BRCA carriers with ovarian, breast, or prostate cancer demonstrated evidence of clinical benefit from olaparib treatment.[38] In a phase II trial of olaparib in BRCA-deficient advanced ovarian cancer, an overall response rate of 33% and a clinical benefit rate of 57.6% were observed at a dose of olaparib of 400 mg BID.[39] Additional PARP-inhibitors are now under development from a number of pharmaceutical companies, and their roles as single-agent therapy and in combination with chemotherapy are being investigated in both BRCA-deficient and spontaneous ovarian cancer.
Other targeted agents in ovarian cancer are also under active investigation, including additional anti-angiogenic agents and agents targeted against pathways such as Notch. Additionally, there has been increasing interest in drugs that target the PI3-kinase (PI3K) / AKT pathway. Data from phase I clinical trials with PI3K/AKT-directed therapies presented at ASCO in 2009 suggest evidence of activity of these agents in ovarian cancer,[40] and phase II trials are currently under development.
Conclusion
Significant advances in the understanding of the pathogenesis and management of both newly diagnosed and recurrent ovarian cancer have occurred over the past few years, making the possibility of better outcomes for women with advanced ovarian cancer a reality. Recent studies have raised the question of the ideal timing of cytoreductive surgery for newly diagnosed disease and demonstrated benefit with alternative weekly paclitaxel dosing, while ongoing studies continue to clarify the roles of IP chemotherapy and biological agents in this setting. In the setting of recurrence, new chemotherapy combinations are being explored, and investigation of the activity of various targeted agents, including anti-angiogenic agents, PARP-inhibitors, and PI3K/AKT pathway inhibitors, remains an area of active interest.
Financial Disclosure:Dr. Matulonis has received clinical research support from Genentech and AstraZeneca.
References:
References
1. Jemal A, Siegel R, Ward E, et al: Cancer statistics, 2009. CA: Cancer J Clin 59(4):225-49, 2009.
2. Ovary. In: American Joint Committee on Cancer: AJCC Cancer Staging Manual. 5th ed., Philadelphia, PA: Lippincott-Raven Publishers; 1997. p. 201-6.
3. National Comprehensive Cancer Network: The NCCN Clinical Practice Guidelines in Oncology Ovarian Cancer (Version 2.2009). Available at: http://www.nccn.org. Accessed January 11, 2010.
4. McGuire WP, Hoskins WJ, Brady MF et al: Cyclophosphamide and cisplatin compared with paclitaxel and cisplatin in patients with stage III and stage IV ovarian cancer. N Engl J Med 334(1):1-6, 1996.
5. Ozols RF, Bundy BN, Greer BE et al: Phase III trial of carboplatin and paclitaxel compared with cisplatin and paclitaxel in patients with optimally resected stage III ovarian cancer: a Gynecologic Oncology Group study. J Clin Oncol 21(17):3194-200, 2003.
6. Levanon K, Crum C, Drapkin R: New insights into the pathogenesis of serous ovarian cancer and its clinical impact. J Clin Oncol 26(32):5284-93, 2008.
7. Fathalla MF: Incessant ovulation--a factor in ovarian neoplasia? Lancet 2(7716):163, 1971.
8. Lee Y, Miron A, Drapkin R et al: A candidate precursor to serous carcinoma that originates in the distal fallopian tube. J Pathol 211(1):26-35, 2007.
9. Griffiths CT: Surgical resection of tumor bulk in the primary treatment of ovarian carcinoma. Natl Cancer Inst Monogr 42:101-4, 1975.
10. Vergote I, Tropacup CG, Amant F et al: EORTC-GCG/NCIC-CTG randomised trial comparing primary debulking surgery with neoadjuvant chemotherapy in stage IIIc-IV ovarian, fallopian tube and peritoneal cancer (OvCa). International Gynecologic Cancer Society 2008 Meeting: Abstract #2008_1767. Available at: http://www.igcs.org/Abstract/meeting_2008_1767.html. Accessed January 11, 2010.
11. Junor EJ, Hole DJ, McNulty L et al: Specialist gynaecologists and survival outcome in ovarian cancer: a Scottish national study of 1866 patients. Br J Obstet Gynaecol 106(11):1130-6, 1999.
12. Katsumata N, Yasuda M, Takahashi F et al: Dose-dense paclitaxel once a week in combination with carboplatin every 3 weeks for advanced ovarian cancer: a phase 3, open-label, randomised controlled trial. Lancet 374(9698):1331-8, 2009.
13. Bookman MA, Brady MF, McGuire WP et al: Evaluation of new platinum-based treatment regimens in advanced-stage ovarian cancer: a phase III trial of the Gynecologic Cancer Intergroup. J Clin Oncol 27(9):1419-25, 2009.
14. Herrstedt J, Huober J, Priou F et al: A randomized, phase III study (AGO-OVAR-9, GINECO-TCG, NSGO-OC-0102): gemcitabine-paclitaxel-carboplatin (TCG) versus paclitaxel-carboplatin (TC) as first-line treatment of ovarian cancer (OC): survival of FIGO stage I-IIA patients. J Clin Oncol 27(18s):Abstr LBA5510, 2009.
15. Pignata S, Scambia G, Savarese A et al: Carboplatin plus paclitaxel (CP) versus carboplatin plus stealth liposomal doxorubicin (CLD) in patients with advanced ovarian cancer (AOC): activity and safety results of the MITO-2 randomized multicenter trial. J Clin Oncol 27(18s):Abstr LBA5508, 2009.
16. Burger RA, Brady MF, Bookman MA et al: Phase III trial of bevacizumab (BEV) in the primary treatment of advanced epithelial ovarian cancer (EOC), primary peritoneal cancer (PPC) or fallopian tube cancer (FTC): A Gynecologic Oncology Group study. J Clin Oncol 28(18s):Abstr LBA1, 2010. Oral presentation accessed online at http://www.asco.org June 23, 2010.
17. ICON7 is a randomised (1:1 ratio), 2 arm, multi-centre, Gynecologic Cancer INterGroup (GCIG) open-label phase III trial designed to evaluate the safety and efficacy of adding bevacizumab, to standard chemotherapy (carboplatin and paclitaxel), in patients with advanced epithelial ovarian or primary peritoneal cancer. Available at: http://www.ctu.mrc.ac.uk/icon7/overview.asp. Accessed January 11, 2010.
18. BIBF 1120 or placebo in combination with paclitaxel and carboplatin in first line treatment of ovarian cancer. Available at: http://www.clinicaltrials.gov; access number NCT01015118. Accessed January 11, 2010.
19. Armstrong DK, Bundy B, Wenzel L et al: Intraperitoneal cisplatin and paclitaxel in ovarian cancer. N Engl J Med 354(1):34-43, 2006.
20. Alberts DS, Liu PY, Hannigan EV et al: Intraperitoneal cisplatin plus intravenous cyclophosphamide versus intravenous cisplatin plus intravenous cyclophosphamide for stage III ovarian cancer. N Engl J Med 26;335(26):1950-5, 1996.
21. Markman M, Bundy BN, Alberts DS et al: Phase III trial of standard-dose intravenous cisplatin plus paclitaxel versus moderately high-dose carboplatin followed by intravenous paclitaxel and intraperitoneal cisplatin in small-volume stage III ovarian carcinoma: an intergroup study of the Gynecologic Oncology Group, Southwestern Oncology Group, and Eastern Cooperative Oncology Group. J Clin Oncol 19(4):1001-7, 2001.
22. National Cancer Institute. NCI Clinical Announcement on Intraperitoneal Therapy for Ovarian Cancer. Available at: http://www.cancer.gov/clinicaltrials/developments/IPchemo-digest. Accessed January 11, 2010.
23. Rao G, Crispens M, Rothenberg ML: Intraperitoneal chemotherapy for ovarian cancer: overview and perspective. J Clin Oncol 25(20):2867-72, 2007.
24. Fujiwara K, Nagao S, Kigawa J et al: Phase II study of intraperitoneal carboplatin with intravenous paclitaxel in patients with suboptimal residual epithelial ovarian or primary peritoneal cancer: a Sankai Gynecology Cancer Study Group Study. Int J Gynecol Cancer 19(5):834-7, 2009.
25. Krasner CN, Seiden MV, Fuller AF et al: Results of all-intraperitoneal carboplatin and paclitaxel regimen shows good tolerability and efficacy for advanced ovarian cancer. J Clin Oncol 25(18s): Abstr 5521, 2007.
26. Rustin GJ, Van der Burg MEL, MRC and EORTC Collaborators. A randomized trial in ovarian cancer (OC) of early treatment of relapse based on CA125 level alone versus delayed treatment based on conventional clinical indicators (MRC OV05/EORTC 55955 trials). J Clin Oncol 27(18s):Abstr 1, 2009.
27. Markman M, Rothman R, Hakes T et al: Second-line platinum therapy in patients with ovarian cancer previously treated with cisplatin. J Clin Oncol 9(3):389-93, 1991.
28. Parmar MK, Ledermann JA, Colombo N et al: Paclitaxel plus platinum-based chemotherapy versus conventional platinum-based chemotherapy in women with relapsed ovarian cancer: the ICON4/AGO-OVAR-2.2 trial. Lancet 361(9375):2099-106, 2003.
29. Gonzalez-Martin AJ, Calvo E et al: Randomized phase II trial of carboplatin versus paclitaxel and carboplatin in platinum-sensitive recurrent advanced ovarian carcinoma: a GEICO (Grupo Espanol de Investigacion en Cancer de Ovario) study. Ann Oncol 16(5):749-55, 2005.
30. Pfisterer J, Plante M, Vergote I et al: Gemcitabine plus carboplatin compared with carboplatin in patients with platinum-sensitive recurrent ovarian cancer: an intergroup trial of the AGO-OVAR, the NCIC CTG, and the EORTC GCG. J Clin Oncol 24(29):4699-707, 2006.
31. Alberts DS, Liu PY, Wilczynski SP et al: Randomized trial of pegylated liposomal doxorubicin (PLD) plus carboplatin versus carboplatin in platinum-sensitive (PS) patients with recurrent epithelial ovarian or peritoneal carcinoma after failure of initial platinum-based chemotherapy (Southwest Oncology Group Protocol S0200). Gynecol Oncol 108(1):90-4, 2008.
32. Pujade-Lauraine E, Mahner S, Kaem J et al: A randomized, phase III study of carboplatin and pegylated liposomal doxorubicin versus carboplatin and paclitaxel in relapsed platinum-sensitive ovarian cancer (OC): CALYPSO study of the Gynecologic Cancer Intergroup (GCIG). J Clin Oncol 27(18s): Abstr LBA5509, 2009.
33. Monk BJ, Herzog T, Kaye S et al: A randomized phase III study of trabectedin with pegylated lioposomal doxorubicin (PLD) versus PLD in relapsed, recurrent ovarian cancer (OC). Ann Oncol 19(8suppl):viii2, LBA4, 2008.
34. Monk BJ, Han E, Josephs-Cowan CA et al: Salvage bevacizumab (rhuMAB VEGF)-based therapy after multiple prior cytotoxic regimens in advanced refractory epithelial ovarian cancer. Gynecol Oncol 102(2):140-4, 2006.
35. Cannistra SA, Matulonis UA, Penson RT et al: Phase II study of bevacizumab in patients with platinum-resistant ovarian cancer or peritoneal serous cancer. J Clin Oncol 25(33):5180-6, 2007.
36. Matulonis UA, Berlin S, Ivy P et al: Cediranib, an oral inhibitor of vascular endothelial growth factor receptor kinases, is an active drug in recurrent epithelial ovarian, fallopian tube, and peritoneal cancer. J Clin Oncol 27(33):5601-6, 2009.
37. Iglehart JD, Silver DP. Synthetic lethality--a new direction in cancer-drug development. N Engl J Med 361(2):189-91, 2009.
38. Fong PC, Boss DS, Yap TA et al: Inhibition of poly(ADP-ribose) polymerase in tumors from BRCA mutation carriers. N Engl J Med 361(2):123-34, 2009.
39. Audeh MW, Penson RT, Friedlander M et al: Phase II trial of the oral PARP inhibitor olaparib (AZD2281) in BRCA-deficient advanced ovarian cancer. J Clin Oncol 27(15s): Abstr 5500, 2009.
40. Wagner AJ, Von Hoff DH, LoRusso PM et al: A first-in-human phase I study to evaluate the pan-PI3K inhibitor GDC-0941 administered QD or BID in patients with advanced solid tumors. J Clin Oncol 27(15s): Abstr 3501, 2009.