New Insights and Emerging Therapies for Breast Cancer Brain Metastases
The diagnosis of central nervous system (CNS) recurrence is a much dreaded outcome among breast cancer patients, and its incidence varies with disease stage and cancer subtype.
MRI image of a human brain shows a bright blue color where brain cancer metastasizes in the occipital lobe.
Breast cancer brain metastases (BCBMs) are the second most frequent secondary central nervous system metastases following those associated with non–small-cell lung cancer. It is increasingly evident that BCBM arises as a function of the biology of the primary tumor and the metastatic niche, which combine to create a unique microenvironment in the brain impacting both metastatic colonization and therapeutic response. Clinical outcomes are improving for BCBM patients as a result of modern combinatorial therapies, challenging the traditionally nihilistic approach to this patient subgroup. This review will focus on the breast cancer subtypes with the highest incidence of BCBM-human epidermal growth factor receptor 2 (HER2)-positive breast cancer, and triple-negative (estrogen receptor [ER]-negative, progesterone receptor [PR]-negative, and HER2-negative) breast cancer (TNBC)-and will characterize differences in the clinical behavior of brain metastases that arise from these different subtypes. We will also highlight some of the recent preclinical studies that may shed light on the biological mechanisms and mediators underlying brain metastases. Finally, we will review published and current prospective trials of systemic therapies specifically for BCBM, including novel pathway-specific therapies.
The Changing Landscape of Breast Cancer Brain Metastases
The diagnosis of central nervous system (CNS) recurrence is a much dreaded outcome among breast cancer patients, and its incidence varies with disease stage and cancer subtype. While less common than bony, lung, or liver metastases, breast cancer brain metastases (BCBMs) are associated with the shortest survival time once diagnosed.[1] BCBMs are also the second most frequent secondary CNS metastases following those associated with non–small-cell lung cancer. BCBMs are typically multifocal and intracerebral, and less commonly solitary and leptomeningeal.[2,3] It is increasingly evident that BCBM arises as a function of the biology of the primary tumor and the metastatic niche; the latter is comprised of the blood-brain barrier (BBB), pericytes, astrocytes, and glial cells, which combine to create a unique microenvironment in the brain that impacts both metastatic colonization and therapeutic response. Improvements in systemic therapy have altered the natural history of breast cancer, and BCBM occurs in a significant proportion of patients. However, patients with BCBM are excluded from many clinical trials, despite the urgent need to develop treatments for this critical challenge. In this article, we will compare the clinical behavior of BCBM associated with the various breast cancer subtypes, with a focus on the subtypes with the highest incidence of BCBM, human epidermal growth factor receptor 2 (HER2)-positive breast cancer and triple-negative (estrogen receptor [ER]-negative, progesterone receptor [PR]-negative, and HER2-negative) breast cancer (TNBC). We will also review therapies and research strategies currently in use and in development.
TABLE 1
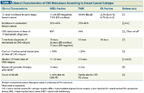
Clinical Characteristics of CNS Metastases According to Breast Cancer Subtype
Differences between the molecular subtypes of breast cancer extend beyond prognosis and therapeutic response to include clinical behavior and patterns of metastatic spread (Table 1). In a study of 3726 patients with early-stage breast cancer diagnosed between 1986 and 1992, the 15-year cumulative incidence rates of brain metastasis were highest in the HER2-positive and TNBC subtypes and lowest in the luminal subtypes.[1] Similar subtype distributions have also been seen in the metastatic setting, where the incidence of BCBM in the HER2-positive and TNBC subtypes is 15% to 44% and 25% to 46%, respectively, with ER negativity and a higher disease burden predicting for a higher BCBM risk in HER2-positive breast cancer.[1,2,4-6] In a separate analysis of 213 patients with BCBM, the median brain metastasis–free survival (BMFS), defined as the time from the diagnosis of extracranial metastases to the time of BCBM, was 34, 18, and 12 months in the ER-positive, HER2-positive, and TNBC subtypes, respectively.[7] In the HER2-positive subgroup, ER co-expression resulted in a prolonged BMFS (26 vs 15 months without ER co-expression). In our series of patients with HER2-positive metastatic breast cancer diagnosed between 1999 and 2005, 8% of patients had BCBM at the time of first metastatic diagnosis, and this increased to 55% with BCBM by the time of death or last follow-up. The BMFS and median survival from the time of BCBM diagnosis were 1.3 and 1.5 years, respectively (Olson EM et al, manuscript in preparation). The variability in the reported median time to CNS progression following diagnosis of HER2-positive metastatic disease may be explained in part by the variable use of HER2-directed therapy in the different studies. Control of extracranial disease at the time of diagnosis of HER2-positive BCBM is approximately 50%, in contrast to TNBC, in which such control is uncommon.[2] Consequently, the cause of death in patients with triple-negative BCBM is rarely due to progressive CNS disease alone, in contrast to HER2-positive BCBM, a setting in which up to 50% of patients die of progressive CNS disease.[2] Concordant with this observation is the longer median overall survival from time of BCBM diagnosis that is seen in the HER2-positive subtype compared with the TNBC subtype (1 to 2 years vs 3 to 5 months).[2-5,8,9] Finally, in a multi-institutional retrospective analysis of patients with newly diagnosed CNS metastases from various primary sites, including 400 patients with breast cancer, univariate and multivariate analyses of prognostic factors associated with outcomes revealed that performance status and tumor subtype were the primary determinants of outcome in BCBM.[10] In contrast, the number of CNS metastases and presence of extracranial metastases were not among the main determinants. Based on the results of their analysis, the authors put forth a disease-specific graded prognostic index for brain metastases, and subsequently refined it further for patients with BCBM; the median survival in the best-graded BCBM group in this index was approximately 25 months.[11]
The natural course of BCBM is thus strongly influenced by the biology of the primary tumor subtype. In HER2-positive disease, some patients experience extended survival relative to historical estimates due to effective targeted therapies in the extracranial setting; multiple lines of CNS-directed therapies are therefore needed, and there is an urgent need to develop therapies that are effective in the HER2-positive CNS. In contrast, patients with TNBC have relatively poorer outcomes, and both CNS and extracranial disease contribute to the shorter survival; thus, there is a need for better systemic treatments targeting all metastatic sites.
Biology of Breast Cancer Brain Metastases
The biological basis of BCBM is largely unknown. Distant metastases in breast cancer have been postulated as an early event, following which the disseminated tumor cells enter a state of proliferative dormancy. The period of metastatic latency is defined by the temporal gap between metastatic infiltration and the acquisition of colonization competency in a distant organ-a competency that may result from progressive malignant evolution of the
tumor and changes in the tumor microenvironment.[12]
The characterization of the molecular subtypes of breast cancer was a major advance in our understanding of the heterogeneity of breast cancer biology. These studies of breast cancer heterogeneity have so far yielded little insight into the different patterns of development of distant metastases across subtypes. One conceptual model classifies genes that underlie the metastatic process into “metastases initiation genes,” which provide transformed cells with the ability to invade and enter the circulatory pathway, and “progression and virulence genes,” which determine the ability to infiltrate and colonize distant organs.[12] Differences in the expression of these genes may underlie the different frequencies of and time to BCBM seen in the different breast cancer subtypes.
Recent breakthroughs in efforts to characterize organ-specific mediators of BCBM include results from gene-expression analyses of BCBM patient samples, which identified cyclooxygenase (COX)-2, epidermal growth factor receptor (EGFR) ligands, and ST6GALNAC5 (a sialyltransferase whose expression is normally restricted to the CNS), as mediators of cancer cell passage through the BBB.[13] In contrast to COX-2 and EGFR, which are also linked to other organ metastases, aberrant expression of ST6GALNAC5 specifically mediated BCBM, potentially by providing a means of enhancing its adhesion to the CNS endothelium and its passage through the BBB. In another study comparing 47 brain metastases and 165 primary breast cancer specimens, the authors found that KISS1 protein, a known metastasis suppressor, was downregulated in metastases compared with primary tumors, and was a prognostic marker for increased risk of breast cancer progression.[14] Finally, an understanding is also emerging of the role of the CNS stroma in the metastatic niche: it appears that reactive glial cells are recruited by highly proliferative CNS metastases, promoting metastatic cell colonization in vivo and supporting tumor cell growth in vitro.[15]
A major hurdle in preclinical brain metastasis research has been the development of suitable models that accurately reflect the biology of BCBM in patients. A unique feature of the CNS is the presence of the BBB, a tight layer of endothelial cells and adipocyte foot processes that acts as a selective barrier to both tumor cell infiltration and the diffusion of systemic therapies, in contrast to the more porous fenestrated endothelia found in bone marrow sinusoids and the liver, which are more readily traversed by circulating tumor cells.[12] The BBB is also characterized by drug efflux mechanisms; for example, preclinical studies in mice have identified a synergistic role between P-glycoprotein and breast cancer resistance protein in modulating the CNS penetration of lapatinib (Tykerb), a small molecule tyrosine kinase inhibitor, under steady-state conditions.[16] There are limited data comparing the concentration of antineoplastic agents in the CNS and in tumor tissue, and there is considerable heterogeneity in the methods by which these data were obtained. A recent overview found CNS-to-blood ratios that, in CNS tumors, were lowest for carboplatin and temozolomide (Temodar), intermediate for paclitaxel and lapatinib, and highest for mitoxantrone.[17] Despite these findings, temozolomide is one of the most effective agents for the treatment of primary CNS tumors; therefore, penetration of the BBB is apparently not always necessary for CNS activity, and the therapeutic effect is also dependent on other properties of the drug and the inherent sensitivity of the tumor. While intrathecal drug administration may represent a potential direct passage into the CNS, this has not routinely been done in the setting of solid malignancies due to the need for reformulation and testing, and the concern that only superficial lesions would be exposed to sufficient drug levels.
Current Treatment Strategies for Breast Cancer Brain Metastases
The National Comprehensive Cancer Network (NCCN) has published guidelines that provide suggested treatment algorithms for patients with CNS metastases from solid tumors.[18] Recently, Kalkanis and colleagues have reformulated consensus guidelines for the management of metastatic brain tumors; standard approaches to symptom management include corticosteroids for the control of peritumoral edema and increased intracranial pressure, and seizure treatment and prevention.[19] While these guidelines represent a significant advance, they are general to all solid tumors, and there are currently none published specifically for the management of BCBM. Most of the cancer therapy recommendations are based on studies in which the majority of patients had non–small-cell lung cancer, and given what we know of the biology of different tumors, it is not clear that these results can be automatically extrapolated to patients with breast cancer.
Local therapies
The management of symptomatic CNS metastases is based on the number, size, and site of lesions, as well as on the status of systemic metastases and patient performance status. The NCCN guidelines for the management of three or fewer CNS lesions (not breast-specific) include surgery and stereotactic radiosurgery (SRS); these approaches are thought to be particularly appropriate for patients with controlled systemic disease. Surgical resection is generally preferred for surgically accessible single CNS metastases, while SRS is an option for patients with lesions in surgically inaccessible locations and for those who are not surgical candidates. Whole brain radiotherapy (WBRT) is generally recommended when there are more than three lesions; WBRT should also be considered (although it is not mandatory) following surgery or SRS, since it has been shown to improve local control but not overall survival.[20] Recent recommendations of the American Society for Radiation Oncology (ASTRO) suggest that SRS may be considered in selected good-prognosis patients with more than three brain metastases. Most randomized controlled trials in patients with CNS metastases include various primary tumors, resulting in few data specific to breast cancer. Unlike with systemic therapies, there are few current published data on the effect of local therapies on BCBM according to breast cancer subtype.
The subject of SRS vs SRS and WBRT is controversial and is beyond the scope of this review. Multiple randomized trials have demonstrated improved intracranial control when WBRT is given following local therapy (ie, SRS and/or surgery).[21-23] At the same time, in a small study, this combination approach was associated with a greater risk of a significant decline in learning and memory function by 4 months compared with SRS alone.[24] However, patients with BCBM are underrepresented in studies of neurocognitive outcomes following local therapy, and there are few long-term outcome data available because of the associated high mortality rate. Potential neurocognitive toxicities resulting from WBRT are increasingly relevant as subgroups of patients with BCBM are experiencing increased median survivals with improved systemic therapies. Since WBRT has not demonstrated an overall survival benefit in this setting, a discussion of the risks and benefits is appropriate, and patients may choose to forgo routine WBRT.
TABLE 2

Prospective Chemotherapy Trials in CNS Metastases
Systemic chemotherapy
Conventional breast cancer chemotherapies are effective in the first-line setting for BCBM, but most of the studies of conventional chemotherapies are older, and the use of adjuvant chemotherapy was not as common then as it is today.[25] As with local therapies, there are few large BCBM-specific prospective systemic therapy trials; most of the relevant trial data are limited to small breast cancer patient cohorts. These trials involve agents such as cisplatin, temozolomide, epothilone B analogues, and combination therapies; CNS objective response rates of up to 40% have been reported in patients with breast cancer (Table 2). First-line therapies typically have better response rates than therapies in heavily pretreated disease, and there is a need to identify therapies that will work in CNS disease that progresses following local and systemic therapies.
Hormonal therapies have also been demonstrated to be effective in ER-positive BCBM. However ER-positive BCBM is relatively uncommon, and many patients have hormone-refractory disease by the time CNS metastases appear, thereby rendering this class of treatment of limited value.
Possible explanations for the failure of systemic treatment include de novo resistance, acquired resistance to prior systemic therapy and radiotherapy, and an inability to penetrate the BBB, resulting in low CNS drug levels. The intrinsic sensitivity of tumor cells to the pharmacologic agent is likely the most important determinant of therapeutic success. Appropriate preclinical efforts are urgently required to address each of these challenges.
HER2-directed therapies
The pattern of disease recurrence in the HER2-positive breast cancer subtype has changed dramatically as a result of the routine use of adjuvant HER2-directed therapy. The use of adjuvant trastuzumab (Herceptin), a recombinant humanized monoclonal antibody directed against the extracellular domain of HER2, has not only been effective in reducing the recurrence rates of HER2-positive breast cancer, but it has also altered the pattern of relapse and survival following the diagnosis of BCBM.[26,27] Interestingly, about half of patients treated with trastuzumab will either be responding to therapy or have stable disease at the time of diagnosis of BCBM; the remainder will die of progressive CNS disease.[5] While trastuzumab is relatively effective in visceral and bony disease, the brain is increasingly recognized as a sanctuary site for tumor cells due to the relative difficulty larger monoclonal antibody therapies have in penetrating the BBB.[5,28] Evidence for this comes from the significantly lower cerebrospinal fluid levels of trastuzumab relative to plasma levels.[6,29] Interestingly, the CSF-to-serum trastuzumab concentration ratio has been shown to be improved in the setting of meningeal disease and WBRT.[6]
TABLE 3
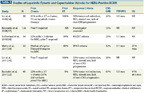
Studies of Lapatinib (Tykerb) and Capecitabine (Xeloda) for HER2-Positive BCBM
Lapatinib is a small molecule tyrosine kinase inhibitor that targets the cytoplasmic ATP-binding sites of the kinase domains of HER2 and EGFR. It is unclear whether lapatinib is able to cross the intact BBB. Unlike trastuzumab, for which the size of the molecule is a major impediment to penetrating the CNS, the mechanism that results in low CNS levels of lapatinib may be at least in part related to the agent’s removal from the CNS by P-glycoprotein, a multidrug transporter.[30] The use of lapatinib is currently FDA-approved in combination with capecitabine (Xeloda), based on a pivotal phase III study of 324 patients with metastatic breast cancer who had received prior anthracycline, taxane, and trastuzumab therapy.[31] In this study, there were statistically fewer CNS progression events in patients treated with the combination than in patients who received capecitabine alone, although the number of events in either arm was small (4 vs 13 events).[32] We conducted two prospective phase II studies of single-agent lapatinib in patients with BCBM, the majority of whom had progressed on ≥ 2 lines of trastuzumab combination therapy and CNS radiotherapy, a common clinical scenario for which the optimal treatment strategy is not defined.[33,34] The objective CNS response rate was 2.6% to 6% and the progression-free survival was 2.6 to 3 months. We subsequently compared lapatinib in combination with capecitabine or topotecan in patients with CNS progression following standard CNS radiotherapy in a phase II trial. The study was closed early due to excess toxicity and lack of efficacy in the lapatinib plus topotecan arm. However, there were promising indications of CNS activity with lapatinib plus capecitabine, with an objective CNS response rate of 38%.[35] This combination has also been studied in a number of other trials (Table 3), none of which included a capecitabine-alone control arm in light of results from the pivotal metastatic breast cancer study.[31] The response criteria differed across trials, and included tumor volume reduction, improvement in neurologic symptoms and signs, reduced corticosteroid usage, and (in some) lack of non-CNS progression. CNS objective response rates with the lapatinib plus capecitabine combination were 18% to 38% in patients who had received prior trastuzumab combination therapies, and up to 67% in a trial in which the majority of patients received this combination as their first metastatic HER2-directed therapy and prior to WBRT.[34-39] In the latter trial, the median time to progression was 5.5 months, and the 1-year overall survival exceeded 70%, supporting the option of delaying WBRT and instead initiating a trial of systemic therapy.[36] Finally, novel HER2-directed therapies currently being studied in phase II trials in HER2-positive BCBM include neratinib and afatinib (ClinicalTrials.gov identifiers: NCT01494662 and NCT01441596, respectively).
Novel systemic and combination therapies
In designing therapies specific for BCBM, the ideal would be one that adequately penetrates the BBB, and targets markers that are specifically expressed by the CNS tumor cells. Novel therapies in development for brain metastases include GRN1005, a peptide (angiopep-2)-taxane conjugate that specifically targets lipoprotein receptor–related protein 1, which is upregulated on various tumor cells and highly expressed on the surface of the BBB, resulting in a CNS uptake that is significantly higher than that seen with other conventional systemic therapies. Phase I results of this compound in a heavily pretreated population with solid tumors and brain metastases revealed an overall partial response and a CNS objective response rate of 25% at the maximum tolerated dose (650 mg/m2); also, 11% of patients who received treatment at 30 to 700 mg/m2 experienced stable disease of ≥ 4 months.[40] A phase II trial with GRN1005 in BCBM is underway, in combination with trastuzumab (for patients with HER2-positive BCBM) or alone (for patients with HER2-negative BCBM) (ClinicalTrials.gov identifier: NCT01480583). Another drug designed to penetrate the BBB is 2B3-101, a glutathione-pegylated liposomal doxorubicin; a phase I study of 2B3-101 is currently enrolling patients with solid tumors with brain metastases and malignant gliomas (ClinicalTrials.gov identifier: NCT01386580). Lastly, TPI-287, a third-generation taxane designed to avoid the multidrug resistance (MDR)-1 protein drug efflux mechanism, has shown promising activity in taxane-resistant preclinical models[41]; TPI-287 is currently being evaluated in a phase II trial of patients with BCBM (ClinicalTrials.gov identifier: NCT01332630).
Bevacizumab (Avastin) is a monoclonal antibody that targets vascular endothelial growth factor (VEGF) and that is effective in treating glioblastoma multiforme (GBM). While there was initial concern about the risk of cerebral hemorrhage when treating GBM with bevacizumab, there are emerging data in the setting of CNS metastases from solid tumors that demonstrate the safety of this agent.[42] Also, while there is still controversy regarding the role of bevacizumab in metastatic breast cancer, clinical trials in BCBM are ongoing. We are currently conducting a phase II trial of carboplatin and bevacizumab with the primary end point of CNS objective response rates in patients with progressive BCBM (ClinicalTrials.gov identifier: NCT01004172).
TABLE 4
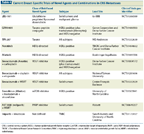
Current Breast-Specific Trials of Novel Agents and Combinations in CNS Metastases
Other therapies for BCBM are developing in parallel with therapies for breast cancer, and involve a number of novel pathways (Table 4). These include the oncogenic PI3K pathway; activating mutations of PIK3CA and/or PTEN loss are commonly found in breast cancer, and have been shown to be active in BCBM.[43] In particular, PIK3CA mutations are found in about one-third of HER2-positive breast cancers and PTEN loss in up to half of TNBC.[44] A phase II single-arm study of everolimus (Afinitor), trastuzumab, and vinorelbine is currently enrolling patients with HER2-positive BCBM, with CNS objective response rates as the primary end point (ClinicalTrials.gov identifier: NCT01305941). Preclinical studies of BKM120, a PI3K inhibitor, demonstrate BBB penetration and efficacy in the treatment of BCBM in mouse models (Zhao J, personal communication). With respect to TNBC, a number of poly (ADP ribose) polymerase (PARP) inhibitors are in various stages of clinical development, and patients carrying BRCA mutations have been shown to be particularly sensitive to treatment with agents in this class. It has been observed that there is a high incidence of BCBM in patients carrying BRCA mutations,[45] and PARP inhibitors currently being assessed in BCBM include ABT-888 (veliparib) in combination with WBRT (ClinicalTrials.gov identifier: NCT00649207). Iniparib, once thought to be a PARP inhibitor, but whose exact mechanism of cytotoxicity is unclear, is being evaluated in a phase II trial in combination with irinotecan (Camptosar) in patients with triple-negative BCBM (ClinicalTrials.gov identifier: NCT01173497). Finally, systemic therapies have also been evaluated as radiosensitizers in combination with WBRT; however, the results of most of these trials have largely been negative. In addition to ABT-888, novel therapies currently being evaluated with WBRT include lapatinib and bevacizumab (ClinicalTrials.gov identifiers: NCT00470847 and NCT01332929, respectively).
Conclusions
CNS metastases are common in patients with the HER2-positive and TNBC breast cancer subtypes, and the natural course of BCBM is strongly influenced by the biology of the primary tumor subtype. The intrinsic sensitivity of tumor cells to various therapies is likely a key determinant of treatment outcomes. Although the biology of BCBM according to tumor subtypes is still poorly understood, recent breakthroughs have been achieved in the identification of specific mediators of CNS metastases and in the development of preclinical models for therapeutic studies. Clinical outcomes are improving for patients with BCBM as a result of modern combinatorial therapies, challenging the traditionally nihilistic approach to this subgroup of patients. Systemic therapies can be effective in BCBM; in particular, effective HER2-directed combination therapies result in prolonged survival in HER2-positive BCBM. However, new treatments are required that effectively target BCBM and systemic metastases in general, particularly in the TNBC subtype. In light of this, reconsideration of the standard eligibility criteria for early-phase trials is needed, since patients with BCBM are routinely excluded on the basis of their historically poor and limited prognosis and the concern that CNS symptoms may complicate the assessment of toxicity.[46]
Because of the exclusion of BCBM patients from more general trials, there has been increasing interest in evaluating novel therapies, both alone and in combination, in BCBM-specific trials. After years of small studies of older chemotherapeutic agents applied unselectively across multiple primary tumor types, we have finally reached an era in which there is demonstrated proof-of-concept that novel targeted agents can produce deep and prolonged responses in some patients with BCBM. While these studies have yet to lead to registration or approval for a BCBM indication, the groundwork is finally being laid for potentially making this a reality in the near future.
Financial Disclosure:Dr. Lim has no significant financial interest or other relationship with the manufacturers of any products or providers of any service mentioned in this article. Dr. Lin has received research funding from GlaxoSmithKline, Genentech, Geron, Boehringer Ingelheim, and Bayer; she serves as a consultant to GlaxoSmithKline, Geron, Novartis, and to-BBB. Dr. Lin also wishes to acknowledge receiving research support from the Breast Cancer Research Foundation.
References:
References
1. Kennecke H, Yerushalmi R, Woods R, et al. Metastatic behavior of breast cancer subtypes. J Clin Oncol. 2010;28:3271-7.
2. Lin NU, Claus E, Sohl J, et al. Sites of distant recurrence and clinical outcomes in patients with metastatic triple-negative breast cancer: high incidence of central nervous system metastases. Cancer. 2008;
113:2638-45.
3. Dawood S, Broglio K, Esteva FJ, et al. Defining prognosis for women with breast cancer and CNS metastases by HER2 status. Ann Oncol. 2008;19:1242-8.
4. Brufsky AM, Mayer M, Rugo HS, et al. Central nervous system metastases in patients with HER2-positive metastatic breast cancer: incidence, treatment, and survival in patients from registHER. Clin Cancer Res. 2011;17:4834-43.
5. Bendell JC, Domchek SM, Burstein HJ, et al. Central nervous system metastases in women who receive trastuzumab-based therapy for metastatic breast carcinoma. Cancer. 2003;97:2972-7.
6. Stemmler HJ, Schmitt M, Willems A, et al. Ratio of trastuzumab levels in serum and cerebrospinal fluid is altered in HER2-positive breast cancer patients with brain metastases and impairment of blood-brain barrier. Anticancer Drugs. 2007;18:23-8.
7. Berghoff A, Bago-Horvath Z, De Vries C, et al. Brain metastases free survival differs between breast cancer subtypes. Br J Cancer. 2012;106:440-6.
8. Niwinska A, Murawska M, Pogoda K. Breast cancer subtypes and response to systemic treatment after whole-brain radiotherapy in patients with brain metastases. Cancer. 2010;116:4238-47.
9. Melisko ME, Moore DH, Sneed PK, et al. Brain metastases in breast cancer: clinical and pathologic characteristics associated with improvements in survival. J Neurooncol. 2008;88:359-65.
10. Sperduto PW, Kased N, Roberge D, et al. Summary report on the graded prognostic assessment: an accurate and facile diagnosis-specific tool to estimate survival for patients with brain metastases. J Clin Oncol. 2012;30:419-25.
11. Sperduto PW, Kased N, Roberge D, et al. Effect of tumor subtype on survival and the graded prognostic assessment for patients with breast cancer and brain metastases. Int J Radiat Oncol Biol Phys. 2012;82:
2111-7.
12. Nguyen DX, Bos PD, Massague J. Metastasis: from dissemination to organ-specific colonization. Nat Rev Cancer. 2009;9:274-84.
13. Bos PD, Zhang XH, Nadal C, et al. Genes that mediate breast cancer metastasis to the brain. Nature. 2009;459:1005-9.
14. Ulasov IV, Kaverina NV, Pytel P, et al. Clinical significance of KISS1 protein expression for brain invasion and metastasis. Cancer. 2012;118:2096-105.
15. Fitzgerald DP, Palmieri D, Hua E, et al. Reactive glia are recruited by highly proliferative brain metastases of breast cancer and promote tumor cell colonization. Clin Exp Metastasis. 2008;25:799-810.
16. Polli JW, Olson KL, Chism JP, et al. An unexpected synergist role of P-glycoprotein and breast cancer resistance protein on the central nervous system penetration of the tyrosine kinase inhibitor lapatinib (N-{3-chloro-4-[(3-fluorobenzyl)oxy]phenyl}-6-[5-({[2-(methylsulfonyl)ethyl]amino}methyl)-2-furyl]-4-quinazolinamine; GW572016). Drug Metab Dispos. 2009;37:439-42.
17. Pitz MW, Desai A, Grossman SA, Blakeley JO. Tissue concentration of systemically administered antineoplastic agents in human brain tumors. J Neurooncol. 2011;104:629-38.
18. National Comprehensive Cancer Network (NCCN) clinical practice guidelines in oncology. Central nervous system cancers. Version I, 2012. Available from: www.nccn.org/professionals/physician_gls/pdf/cns.
19. Kalkanis SN, Linskey ME. Evidence-based clinical practice parameter guidelines for the treatment of patients with metastatic brain tumors: introduction. J Neurooncol. 2010;96:7-10.
20. Aoyama H, Tago M, Kato N, et al. Neurocognitive function of patients with brain metastasis who received either whole brain radiotherapy plus stereotactic radiosurgery or radiosurgery alone. Int J Radiat Oncol Biol Phys. 2007;68:1388-95.
21. Kocher M, Soffietti R, Abacioglu U, et al. Adjuvant whole-brain radiotherapy versus observation after radiosurgery or surgical resection of one to three cerebral metastases: results of the EORTC 22952-26001 study. J Clin Oncol. 2011;29:134-41.
22. Patchell RA, Tibbs PA, Walsh JW, et al. A randomized trial of surgery in the treatment of single metastases to the brain. N Engl J Med. 1990;322:494-500.
23. Aoyama H, Shirato H, Tago M, et al. Stereotactic radiosurgery plus whole-brain radiation therapy vs stereotactic radiosurgery alone for treatment of brain metastases: a randomized controlled trial. JAMA. 2006;295:2483-91.
24. Chang EL, Wefel JS, Hess KR, et al. Neurocognition in patients with brain metastases treated with radiosurgery or radiosurgery plus whole-brain irradiation: a randomised controlled trial. Lancet Oncol. 2009;
10:1037-44.
25. Rosner D, Nemoto T, Lane WW. Chemotherapy induces regression of brain metastases in breast carcinoma. Cancer. 1986;58:832-9.
26. Piccart-Gebhart MJ, Procter M, Leyland-Jones B, et al. Trastuzumab after adjuvant chemotherapy in HER2-positive breast cancer. N Engl J Med. 2005;
353:1659-72.
27. Romond EH, Perez EA, Bryant J, et al. Trastuzumab plus adjuvant chemotherapy for operable HER2-positive breast cancer. N Engl J Med. 2005;353:1673-84.
28. Burstein HJ, Lieberman G, Slamon DJ, et al. Isolated central nervous system metastases in patients with HER2-overexpressing advanced breast cancer treated with first-line trastuzumab-based therapy. Ann Oncol. 2005;16:1772-7.
29. Pestalozzi BC, Brignoli S. Trastuzumab in CSF. J Clin Oncol. 2000;18:2349-51.
30. Polli JW, Humphreys JE, Harmon KA, et al. The role of efflux and uptake transporters in [N-{3-chloro-4-[(3-fluorobenzyl)oxy]phenyl}-6-[5-({[2-(methylsulfonyl)ethyl]amino}methyl)-2-furyl]-4-quinazolinamine (GW572016, lapatinib) disposition and drug interactions. Drug Metab Dispos. 2008;36:695-701.
31. Geyer CE, Forster J, Lindquist D, et al. Lapatinib plus capecitabine for HER2-positive advanced breast cancer. N Engl J Med. 2006;355:2733-43.
32. Geyer CE, Martin A, Newstat B, et al. Lapatinib (L) plus capecitabine (C) in HER2+ advanced breast cancer (ABC): updated efficacy and biomarker analysis.
J Clin Oncol 2007;25:(abstr 1035).
33. Lin NU, Carey LA, Liu MC, et al. Phase II trial of lapatinib for brain metastases in patients with human epidermal growth factor receptor 2-positive breast cancer. J Clin Oncol. 2008;26:1993-9.
34. Lin NU, Dieras V, Paul D, et al. Multicenter phase II study of lapatinib in patients with brain metastases from HER2-positive breast cancer. Clin Cancer Res. 2009;15:1452-9.
35. Lin NU, Eierman W, Greil R, et al. Randomized phase II study of lapatinib plus capecitabine or lapatinib plus topotecan for patients with HER2-positive breast cancer brain metastases. J Neurooncol. 2011;
105:613-20.
36. Bachelot TD, Romieu G, Campone M, et al. LANDSCAPE: An FNCLCC phase II study with lapatinib (L) and capecitabine (C) in patients with brain metastases (BM) from HER2-positive (+) metastatic breast cancer (MBC) before whole-brain radiotherapy (WBR). J Clin Oncol. 2011;29(suppl; abstr 509).
37. Boccardo F, Kaufman B, Baselga J, et al. Evaluation of lapatinib (Lap) plus capecitabine (Cap) in patients with brain metastases (BM) from HER2+ breast cancer (BC) enrolled in the Lapatinib Expanded Access Program (LEAP) and French Authorisation Temporaire d'Utilisation (ATU). J Clin Oncol. 2008;26(abstr 1094).
38. Sutherland S, Ashley S, Miles D, et al. Treatment of HER2-positive metastatic breast cancer with lapatinib and capecitabine in the lapatinib expanded access programme, including efficacy in brain metastases-the UK experience. Br J Cancer. 2010;102:995-1002.
39. Metro G, Foglietta J, Russillo M, et al. Clinical outcome of patients with brain metastases from HER2-positive breast cancer treated with lapatinib and capecitabine. Ann Oncol. 2011;22:625-30.
40. Kurzrock R, Gabrail N, Chandhasin C, et al. Safety, pharmacokinetics, and activity of GRN1005, a novel conjugate of angiopep-2, a peptide facilitating brain penetration, and paclitaxel, in patients with advanced solid tumors. Mol Cancer Ther. 2012;11:308-16.
41. Silberman S, Hwang JH, Marshall JL, et al. A phase I study of TPI 287, a novel taxane, administered weekly in patients with advanced cancer. J Clin Oncol. 2008;26(May 20 suppl; abstr 2536).
42. Besse B, Lasserre SF, Compton P, et al. Bevacizumab safety in patients with central nervous system metastases. Clin Cancer Res. 2010;16:269-78.
43. Adamo B, Deal AM, Burrows E, et al. Phosphatidylinositol 3-kinase pathway activation in breast cancer brain metastases. Breast Cancer Res. 2011;13:R125.
44. Gonzalez-Angulo AM, Ferrer-Lozano J, Stemke-Hale K, et al. PI3K pathway mutations and PTEN levels in primary and metastatic breast cancer. Mol Cancer Ther. 2011;10:1093-101.
45. Tischkowitz MD, Foulkes WD. The basal phenotype of BRCA1-related breast cancer: past, present and future. Cell Cycle. 2006;5:963-7.
46. Carden CP, Agarwal R, Saran F, Judson IR. Eligibility of patients with brain metastases for phase I trials: time for a rethink? Lancet Oncol. 2008;9:1012-7.
47. Siena S, Landonio G, Beaietta E. Multicenter phase II study of temozolomide therapy for brain metastasis in patients with malignant melanoma, breast cancer, and non-small cell lung cancer. Proc Am Soc Clin Oncol. 2003;22:(abstract 407).
48. Trudeau ME, Crump M, Charpentier D, et al. Temozolomide in metastatic breast cancer (MBC): a phase II trial of the National Cancer Institute of Canada - Clinical Trials Group (NCIC-CTG). Ann Oncol. 2006;17:952-6.
49. Franciosi V, Cocconi G, Michiara M, et al. Front-line chemotherapy with cisplatin and etoposide for patients with brain metastases from breast carcinoma, non-small cell lung carcinoma, or malignant melanoma: a prospective study. Cancer. 1999;85:1599-605.
50. Christodoulou C, Bafaloukos D, Linardou H, et al. Temozolomide (TMZ) combined with cisplatin (CDDP) in patients with brain metastases from solid tumors: a Hellenic Cooperative Oncology Group (HeCOG) Phase II study. J Neurooncol. 2005;71:61-5.
51. Rivera E, Meyers C, Groves M, et al. Phase I study of capecitabine in combination with temozolomide in the treatment of patients with brain metastases from breast carcinoma. Cancer. 2006;107:1348-54.
52. Murphy C, Nulsen B, Rump M, et al, editors. Phase II trial of patupilone in patients (pts) with breast cancer brain metastases (BCBM) progressing or recurring after whole brain radiotherapy (WBXRT). ASCO Breast Cancer Symposium; 2009.
53. Freedman RA, Bullitt E, Sun L, et al. A phase II study of sagopilone (ZK 219477; ZK-EPO) in patients with breast cancer and brain metastases. Clin Breast Cancer. 2011;11:376-83.