Provocative Pearls in Diagnosing and Treating Acute Promyelocytic Leukemia
The treatment of APL in the modern era is a success of modern hematology. In this review we have attempted to plant the seeds of understanding regarding how diagnosis and treatment of APL will be pursued over the next decade.
The introduction of all-trans retinoic acid (ATRA) into routine clinical practice changed the outcome of acute promyelocytic leukemia (APL) from the most fatal to the most curable subtype of acute myeloid leukemia (AML). Patients who do not survive generally succumb within the first 30 days after presentation or diagnosis, often from intracranial or pulmonary hemorrhage caused by the characteristic coagulopathy associated with this disease. For the majority of patients who avoid hemorrhagic complications, the goals of decreasing the side effects of diagnosis and treatment-including pain, inpatient hospital days, and late sequelae of cytotoxic chemotherapy-have emerged as paramount. Here, we discuss novel and provocative observations regarding diagnostic and treatment strategies for APL that are likely to emerge as standards of care in the next 5 years, and that may improve the rate of early hemorrhagic death and decrease diagnosis- and treatment-related morbidity.
Introduction
The treatment of acute promyelocytic leukemia (APL) is a success story of modern hematology. Characterized by a translocation fusing the promyelocytic leukemia gene (PML) on chromosome 15 with the retinoic acid receptor alpha gene (RARα) on chromosome 17, myeloid precursors in APL are blocked from differentiating past the promyelocyte stage, with subsequent inhibition of normal hematopoiesis. After introduction of all-trans retinoic acid (ATRA) into routine clinical practice, the survival of patients with APL dramatically improved. Indeed, in the first North American Intergroup trial (I0129), patients who received ATRA during both induction and maintenance had a disease-free survival rate of approximately 75%.[1] Subsequent refinement of ATRA treatment schedules and the introduction of arsenic trioxide (ATO) allow cure of the majority of patients with APL. Patients who survive the first 30 days after diagnosis of their disease, when coagulopathy and the ATRA differentiation syndrome can cause morbidity and mortality, have an extraordinarily high rate of cure.
Given that most patients now survive and are cured, research efforts have shifted towards strategies to minimize early death and toxicity from treatment. In this brief review we address new “provocative pearls” in the diagnosis and treatment of APL. After discussing the generally agreed-upon international guidelines for treatment, we focus on the next generation of diagnostic and treatment strategies.
International Guidelines for Management of APL
ATRA was introduced into clinical practice in China in the early 1990s, and the first intergroup trial performed by the North American Intergroup was reported in 1997.[1] This trial randomized APL patients to induction with ATRA or chemotherapy (daunorubicin and cytarabine [Ara- C]). Once remission was achieved, a second randomization assigned patients to ATRA maintenance or observation alone. The results were striking and anticipated the current age of targeted agents in cancer therapy. Clinically and statistically significant increases in overall and disease-free survival were observed among the patients who received ATRA, in either induction or maintenance, compared with the chemotherapy groups. Since 1997, findings from multiple welldesigned clinical trials in the United States, Europe, and elsewhere have led to an increase in the cure rate of APL, to more than 80%.[2-5]
Current guidelines advocate establishing a diagnosis of APL based on the morphology of a bone marrow aspirate and finding the characteristic translocation between chromo- somes 15 and 17 by cytogenetics, fluorescence in situ hybridization (FISH), or molecular testing. Patients are then risk-stratified based on their presentation white blood cell (WBC) count; those with a WBC count greater than 10,000/μL are considered high risk, and those with a WBC count lower than 10,000/μL are at either low or intermediate risk (based on the platelet count). Clinically, the low-/ intermediate-risk categories are now grouped together and receive identical treatment. As most regimens for APL include an anthracycline in induction and consolidation, the first clinical decision for practicing oncologists is whether the individual patient can tolerate an anthracycline. Patients with overt heart failure or borderline cardiac function, as well as elderly patients who may not be able to tolerate cytotoxic chemotherapy, should have an anthracycline omitted from their treatment regimen and can be given a combination of ATRA and ATO, which has shown excellent cure rates in phase II clinical trials. Patients who are able to tolerate anthracyclines, who have either high-risk or low-risk disease, are treated with ATRA at a dose of 45 mg/m2 in combination with an anthracycline, or ATRA at 45 mg/m2 in combination with an anthracycline and cytarabine, respectively. A number of cooperative groups and practicing physicians now omit cytarabine from the treatment of patients with low-risk disease. Following induction, consolidation is given for 2 to 3 cycles and incorporates ATRA with cytotoxic chemotherapy. There are multiple consolidation chemotherapy regimens; these reflect the different treatment strategies undertaken by different cooperative groups in clinical trials performed in Europe, the United States, and other parts of the world. The role of maintenance therapy, particularly in low-risk disease, continues to be a matter of active clinical investigation, but most experts agree that a combination of 6-mercaptopurine, methotrexate, and ATRA should be given to patients with high-risk disease.
Pearls in Diagnosis, Complete Remission, and Prognosis
For many patients, the most difficult part of the initial evaluation for acute leukemia is a bone marrow aspiration and biopsy. Even with adequate local anesthesia, many people experience significant procedural discomfort that leads to subsequent anxiety and in some cases, refusal of further bone marrow evaluation. There are several reasons for performing bone marrow biopsy in AML: assessment of bone marrow blast percentage (which forms the basis for a firm diagnosis of AML); evaluation of bone marrow morphology; use of immunohistochemical staining to assess leukemic lineage (lymphoid or myeloid); and the ability to obtain adequate numbers of myeloblasts for cytogenetic and, to a lesser extent, molecular genetic analysis. In AML, the initial bone marrow biopsy is crucial because the results of the cytogenetic and molecular genetic studies form the basis of treatment recommendations in consolidation-specifically regarding whether to proceed to allogeneic stem cell transplantation, or consolidate with multiple courses of high-dose cytarabine or a similar regimen.
However, APL has unique associations that raise the question of whether a bone marrow biopsy at diagnosis is actually needed. The morphology of leukemic promyelocytes (if they are present in the peripheral blood) is distinctive and consists of promyelocytes with abundant granules that form bundles resembling collections of sticks (so called faggot cells). In addition, the presence of the t(15;17) translocation, identified by cytogenetics, FISH, or molecular genetics, is unique to APL and confirms the diagnosis. Often, review of the peripheral blood in patients with APL reveals the characteristic leukemic promyelocytes, and if these are present, the cytogenetics are also clear. Although additional cytogenetic abnormalities aside from t(15;17) exist in about one-third of patients with APL, these have not been consistently associated with disease outcome and are not included in risk stratification. In fact, the prognosis is based solely on peripheral WBC count and age, and as mentioned earlier, treatment strategy is based on risk status and ability to tolerate anthracyclines. Novel molecular genetic abnormalities have an important influence on prognosis in other subtypes of AML, but no clear influence in treatment and outcome in APL.
In other forms of AML, a day 14 nadir bone marrow aspirate and biopsy are obtained. In APL this is not needed because it often takes more than 14 days for the leukemic promyelocytes to differentiate. Furthermore, a day 14 marrow in APL is characteristically replete with differentiating leukemia cells, and there is very often no typical marrow aplasia. As the incidence of primary ATRA resistance during induction therapy is vanishingly small, a patient whose peripheral blood counts normalize with ATRA plus anthracycline-based therapy can be presumed to be in a complete remission (CR). An initial CR can be verified using sensitive molecular genetic tests for the PML-RAR fusion product in peripheral blood. Therefore, it may be that a bone marrow aspirate and biopsy in first CR are not needed. Once a CR is confirmed, periodic monitoring with molecular genetic testing of peripheral blood are all that are needed to confirm continued disease remission. Thus, it is conceivable that in many patients for whom the diagnosis is clear from the history, physical examination, and laboratory studies, a bone marrow aspirate and biopsy are not needed at diagnosis, at the nadir, or once the patient enters a hematologic CR.
APL is also distinguished from other subtypes of AML in that there are no modifications in therapeutic approach, even in patients with additional cytogenetic abnormalities, therapy-related disease, the FLT3-ITD mutation (commonly present in APL), the PML isoform, and the microgranular variant (M3V).
TABLE 1
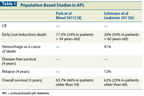
Population-Based Studies in APL
Pearls in Minimizing Early Death
FIGURE 1
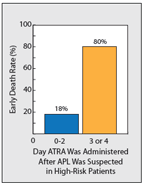
Outcome of Timing of ATRA Administration on High-Risk Patients With APL.
While the cure rates for APL are remarkable, early death (defined as death within 30 days of diagnosis) continues to be a major cause of treatment failure. In clinical trials, the induction death rate ranges between 5% and 9%.[1,4] In population-based studies (including patients who never enrolled in trials), the early death rate ranges between 17% and 30% and is considerably higher in older patients (Table 1).[2,6] Indeed, the early death rate has not changed significantly since the introduction of ATRA. The major cause of early death is hemorrhage, usually pulmonary or intracerebral, caused by the characteristic coagulopathy associated with this disease. What accounts for early death? An abstract presented at the 2011 meeting of the American Society of Hematology suggested that early death may be related to delays in receiving ATRA once patients present to the hospital.[7] The authors hypothesized that early death could be reduced with the rapid administration of ATRA-without waiting for the results of bone marrow aspiration and confirmation of t(15;17). In this retrospective analysis of 194 patients, most patients (69%) had ATRA administered 2 days or more after presentation (Figure 1). While the early death rate was not increased, the percentage of patients who died from hemorrhage was markedly increased when ATRA was delayed for more than 2 days. Results of this retrospective analysis also confirmed that high-risk patients with APL who received their first dose of ATRA 3 or 4 days after they were suspected of having APL had an early death rate of 80%, compared with a rate of only 18% in high-risk patients who received ATRA on days 0, 1, or 2.
While these results are preliminary and need to be confirmed in larger series, we advocate administration of ATRA at the earliest suspicion of APL. ATRA is a relatively innocuous drug with few side effects. If the diagnosis of APL is not confirmed, ATRA can be discontinued. This requires vigilance by hematologists and medical oncologists, and participation and awareness of emergency department and general internal medicine physicians, who often have the first clinical contact with newly diagnosed patients. Also, hospital pharmacies should have ATRA readily available or the ability to obtain a supply of ATRA rapidly. It may be that further improvement in the outcome of APL may come from aggressive blood product support and early administration of ATRA rather than identification of new treatments for the disease.
Pearls in Preventing APL Differentiation Syndrome
After administering ATRA at the first clinical suspicion of APL, the astute physician must be vigilant for the APL differentiation syndrome. The differentiation syndrome, first described with the use of ATRA but also seen with the use of ATO, is manifested clinically as noncardiogenic pulmonary edema that can cause respiratory failure requiring intubation and mechanical ventilation. Its etiology is poorly understood. It is thought to be caused by a capillary leak syndrome induced by the rapid differentiation of leukemic promyelocytes. The treatment, dexamethasone at 10 mg twice daily, should be prescribed immediately to patients who are thought to be developing the differentiation syndrome. In our practice, we administer dexamethasone prophylactically for 10 to 14 days to patients with high-risk disease. APL differentiation syndrome is an example of a situation in which a complication of treatment should be addressed at the very first suspicion and before its diagnosis is established.
Pearls in Maintenance
The question of whether to treat APL like other forms of AML and omit maintenance treatment after consolidation chemotherapy or to treat it instead like acute lymphoblastic leukemia (ALL) and prescribe maintenance therapy continues to be a matter of debate. As part of the AIDA 0493 trial, patients who were in a molecular CR after three courses of consolidation were randomized into one of four maintenance treatment arms: oral 6-MP at a dose of 90 mg/m2/d and intramuscular methotrexate at 15 mg/ m2/week, ATRA alone at a dose of 45 mg/m2 for 15 days every 3 months, alternating chemotherapy (with 6-MP and methotrexate) and ATRA, and observation.[ 8] After January 1997, the chemotherapy-only and observation arms were dropped and patients were randomized to one of the two ATRA treatment arms. When all four arms were analyzed, patients in the observation arm fared worse than those in the ATRA maintenance arms. Patients in both ATRA maintenance arms had similar rates of disease-free survival. In contrast, the APL97 study of the Japanese Acute Leukemia Study Group (JALSG) randomized patients who were in a molecular remission to 3 cycles of intensified maintenance chemotherapy or observation.[9] Patients who were molecularly positive were given ATRA for 4 weeks followed by the same 3 cycles of chemotherapy. Interestingly, disease-free survival did not differ significantly between the maintenance and no-maintenance groups, but overall survival was significantly worse in the maintenance chemotherapy arm.
The role of maintenance, therefore, is currently unclear. Our general recommendations are that patients should receive maintenance therapy, particularly if they are high-risk at the time of diagnosis or develop an elevated WBC count during treatment.
TABLE 2
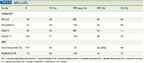
ATO in APL
Curing Patients Without Chemotherapy
The side effects of chemotherapy used in APL-cytopenias, neutropenic fever, and late cardiac toxicity-are significant. The holy grail of cancer therapy is to achieve remission and cure without the use of cytotoxic chemotherapy. In APL, this goal may be achievable. There are now a number of well-designed clinical trials demonstrating the efficacy of a combination of ATRA and ATO or even ATO alone (in very-lowrisk disease) used in induction, consolidation, or both (Table 2).[10,11]
Hu and colleagues treated 85 patients from April 2001 to December 2005 with an induction regimen of ATRA and ATO daily until documentation of CR.[12] Patients who developed WBC counts greater than 10,000/ μL before or during treatment were also given chemotherapy. After CR, patients received three courses of consolidation treatment with chemotherapy alone (no ATRA). They then received 5 cycles of maintenance therapy with ATRA/ ATO and low-dose chemotherapy. The trial showed an impressive 5-year survival rate of 91.7%.
Ravandi and colleagues, in 2009, published the updated results of a clinical trial of ATRA and ATO performed at the MD Anderson Cancer Center. [13] In this trial, 82 patients were given ATRA and ATO induction with the addition of gemtuzumab ozogamicin (Mylotarg) if they had high-risk disease at presentation, or if their WBC count increased to 30,000/μL or greater during treatment. Post-induction therapy was 7 cycles of consolidation that consisted of 4 cycles of combined ATRA and ATO and 3 cycles of ATRA alone. As with Hu's study, the results were impressive. The overall response rate of the entire group was 92%. Overall survival at 5 years was approximately 80%. However, the ability to replicate this study is limited because gemtuzumab ozogamicin was withdrawn from the commercial market because of increased liver toxicity seen in a Southwest Oncology Group trial, with no obvious clinical benefit.
Iland and colleagues have reported the results of the APML 4 study conducted by the Australasian Leukaemia and Lymphoma Group.[11] This study gave all patients a combination of ATRA, ATO, and the standard four doses of idarubicin in induction, followed by 2 cycles of consolidation with ATRA and ATO, followed by standard maintenance therapy with 6-MP, methotrexate, and ATRA given once every 3 months for 2 years. Of the 124 patients evaluated, the 5-year overall survival rate was 93%, and relapse-free survival was a remarkable 98%.
Mathews in 2010 reported the long-term outcomes of a trial of arsenic monotherapy induction (although chemotherapy was allowed at the investigators' discretion to control elevated WBC counts) followed by 4 weeks of ATO consolidation, followed by 10 days of ATO once a month for 6 months as maintenance therapy.[14] The 5-year overall survival in the cohort of 72 patients enrolled on the trial was 74.1%. In a “good-risk” group, defined by the authors as patients presenting with a WBC count lower than 5,000/μL and platelet count higher than 20,000/μL, the overall survival at 5 years was 100%. The event-free survival at 5 years was 90% in the low-risk group and 60% in the high-risk group.
Ghavazmadeh and colleagues subsequently reported results of a phase II trial of 197 patients treated with arsenic monotherapy in post-induction and consolidation treatment.[15] Overall survival was 64.4% at 5 years.
What can we learn from these trials? It seems clear that arsenic monotherapy for induction and consolidation is not sufficient to produce the high overall survival seen for the majority of patients treated with ATRA and chemotherapy. However, a combination of ATRA and ATO without chemotherapy or with minimal chemotherapy to control the WBC count may be effective. There are active clinical trials and trials in development to further study the use of ATRA and ATO without chemotherapy. Therefore, APL represents both the only subtype of AML for which current therapy is directed at less cytotoxic chemotherapy and the only subtype that can be cured with minimal and, in fact, no chemotherapy.
APL in Older Adults
Several recent cooperative group studies demonstrate that the CR rate among older adults with APL is as high as that among younger patients (approximately 85%). Similarly, the relapse rate is quite low, at 10% to 20%, and the early death rate is approximately 15%. Therefore, APL appears to be one of the few subtypes of AML in which the disease appears as sensitive among older adults as it is among younger patients. Furthermore, APL is one of the few subtypes of AML in which improvements in outcome among older adults may depend more on decreasing toxicity than on increasing the amount of anti-leukemia therapy.
Oral Arsenic Trioxide
Introducing oral ATO into routine clinical practice follows the general trend in hematology and medical oncology to deliver oral medications in place of IV formulations. In addition to the convenience for patients, the benefits of oral arsenic are a reduction in the need for long-term venous access devices such as medi-ports and PICC lines. These permanent-access devices are associated with risks of infection and of deep and superficial vein thromboses, with concomitant health care costs associated with inpatient hospital stays, IV antibiotics, and therapeutic anticoagulation.
The safety and efficacy of oral arsenic have been investigated in prior studies undertaken in China. Lu et al published the clinical results and safety data from 129 patients with newly diagnosed or relapsed APL treated from 1994 to 2000 using oral arsenic at a dose of 50 mg/kg per day, divided into four doses, until the patients had a documented hematologic complete remission.[16] Although not directly compared, complete remission and disease-free survival rates were similar to those seen in studies of IV arsenic. Oral arsenic was well tolerated, with the most frequent adverse events being asymptomatic prolongation of QTc (33%), transaminitis (10.5%), gastrointestinal discomfort (3.2%), rash (3.2%), and pericardial effusion (1%), side effects that are also typical of IV administration. Of note, despite the prolongation in QTc, arsenic continued to be administered without any evidence of ventricular arrhythmia.
In a separate report by Au et al, 12 consecutive patients with relapsed APL were treated with single-agent oral arsenic in a formulation slightly different from that used by Lu et al (As2O3 vs As4S4).[17] All patients achieved a CR. The most frequent adverse events were leukocytosis (related to administration when patients had initial relapsed disease), liver function abnormalities, and skin rash. The liver function abnormalities resolved with temporary cessation of treatment, and patients were successfully re-challenged with oral arsenic.
Clinical trials are evaluating the use of oral arsenic in combination with ATRA. Should this combination therapy be successful, APL will become the only subtype of acute leukemia that can be cured with all-oral therapy.
Conclusion
The treatment of APL in the modern era is a success of modern hematology. In this review we have attempted to plant the seeds of understanding regarding how diagnosis and treatment of APL will be pursued over the next decade. It is our hope that this will include rapid diagnostic assessment with a simple venipuncture, swift administration of ATRA- and ATO-based therapies that minimize exposure to cytotoxic chemotherapy, and the performance of well-designed clinical trials that elucidate the role of maintenance in APL treatment.
Financial Disclosure:The authors have no significant financial interest or other relationship with the manufacturers of any products or providers of any service mentioned in this article.
References:
References
1. Tallman MS, Andersen JW, Schiffer CA, et al. Alltrans- retinoic acid in acute promyelocytic leukemia. N Engl J Med. 1997;337:1021-8.
2. Sanz MA, Martin G, Rayon C, et al. A modified AIDA protocol with anthracycline-based consolidation results in high antileukemic efficacy and reduced toxicity in newly diagnosed PML/RARalpha-positive acute promyelocytic leukemia. PETHEMA group. Blood. 1999;94:3015-21.
3. Sanz MA, Montesinos P, Rayon C, et al. Risk-adapted treatment of acute promyelocytic leukemia based on all-trans retinoic acid and anthracycline with addition of cytarabine in consolidation therapy for high-risk patients: further improvements in treatment outcome. Blood. 2010;115:5137-46.
4. Lo-Coco F, Avvisati G, Vignetti M, et al. Front-line treatment of acute promyelocytic leukemia with AIDA induction followed by risk-adapted consolidation for adults younger than 61 years: results of the AIDA-2000 trial of the GIMEMA Group. Blood. 2010;116:3171-9.
5. Ades L, Chevret S, Raffoux E, et al. Is cytarabine useful in the treatment of acute promyelocytic leukemia? Results of a randomized trial from the European Acute Promyelocytic Leukemia Group. J Clin Oncol. 2006;24:5703-10.
6. Lehmann S, Ravn A, Carlsson L, et al. Continuing high early death rate in acute promyelocytic leukemia: a population-based report from the Swedish Adult Acute Leukemia Registry. Leukemia. 2011;25:1128-34.
7. Altman JK, Rademaker A, Cull E, et al. Administration of all-trans retinoic acid (ATRA) to newly diagnosed patients (pts) with acute promyelocytic leukemia (APL) is delayed even at experienced centers and associated with an increased early death rate (EDR): a retrospective analysis of 205 pts. ASH Annual Meeting Abstracts. 2011;118:abstr 942.
8. Avvisati G, Lo-Coco F, Paoloni FP, et al. AIDA 0493 protocol for newly diagnosed acute promyelocytic leukemia: very long-term results and role of maintenance. Blood. 2011;117:4716-25.
9. Asou N, Kishimoto Y, Kiyoi H, et al. A randomized study with or without intensified maintenance chemotherapy in patients with acute promyelocytic leukemia who have become negative for PML-RARalpha transcript after consolidation therapy: the Japan Adult Leukemia Study Group (JALSG) APL97 study. Blood. 2007;110:59-66.
10. Dai CW, Zhang GS, Shen JK, et al. Use of all-trans retinoic acid in combination with arsenic trioxide for remission induction in patients with newly diagnosed acute promyelocytic leukemia and for consolidation/maintenance in CR patients. Acta Haematologica. 2009;121:1-8.
11. Iland HJ, Bradstock K, Supple SG. All-trans-retinoic acid, idarubicin, and intravenous arsenic trioxide as initial therapy in acute promyelocytic leukemia (APML4); Blood. 2012 Jun 19. [Epub ahead of print]
12. Hu J, Liu YF, Wu CF, et al. Long-term efficacy and safety of all-trans retinoic acid/arsenic trioxide-based therapy in newly diagnosed acute promyelocytic leukemia. Proc Natl Acad Sci USA. 2009;106:3342-7.
13. Ravandi F, Estey E, Jones D, et al. Effective treatment of acute promyelocytic leukemia with all-transretinoic acid, arsenic trioxide, and gemtuzumab ozogamicin. J Clin Oncol. 2009;27:504-10.
14. Mathews V, Balasubramanian P, Shaji RV, et al. Arsenic trioxide in the treatment of newly diagnosed acute promyelocytic leukemia: a single center experience. Am J Hematol. 2002;70:292-9.
15. Ghavamzadeh A, Alimoghaddam K, Rostami S, et al. Phase II study of single-agent arsenic trioxide for the front-line therapy of acute promyelocytic leukemia. J Clin Oncol. 2011;29:2753-7.
16. Lu DP, Qiu JY, Jiang B, et al. Tetra-arsenic tetra-sulfide for the treatment of acute promyelocytic leukemia: a pilot report. Blood. 2002;99:3136-43.
17. Au WY, Kumana CR, Kou M, et al. Oral arsenic trioxide in the treatment of relapsed acute promyelocytic leukemia. Blood. 2003;102:407-8.
18. Park JH, Qiao B, Panageas KS, et al. Early death rate in acute promyelocytic leukemia remains high despite all-trans retinoic acid. Blood. 2011;118:1248-54.