Optimizing Adjuvant Chemotherapy in Early-Stage Breast Cancer
Mortality in breast cancer has declined in the past decade, owing toadvances in diagnosis, surgery, radiotherapy, and systemic treatments.Adjuvant chemotherapy has had a major effect on increasing survivalin women with locoregional breast cancer. Like all treatments, adjuvantchemotherapy is a work in progress, and it has evolved from singleoral agents to complex multidrug regimens. The choice of regimens isnot without controversy, however, and several have been shown to bemore effective than others, especially in patients who are at high riskfor recurrence. The taxanes paclitaxel and docetaxel (Taxotere) havebeen shown to be effective in the adjuvant setting, and they have alsobeen shown to improve the outcomes in node-positive disease. Bothdisease-free and overall survival are greater with doxorubicin,paclitaxel, and cyclophosphamide given in a dose-dense, every-2-weekschedule with growth factor support than with the same agents givenin an every-3-week schedule. Disease-free and overall survival in patientswith node-positive disease are greater with docetaxel, doxorubicin(Adriamycin), and cyclophosphamide (TAC) than with fluorouracil,doxorubicin, and cyclophosphamide (FAC). Febrile neutropenia iscommon with the TAC regimen, but it can be minimized with growthfactor support. Based on these findings, dose-dense therapy and TAC arethe current adjuvant treatments of choice in patients with node-positivedisease; other, less-intense regimens may be appropriate in patientswith lower-risk disease. Ongoing trials are investigating the efficacy ofcommonly used regimens, new chemotherapeutic and biologic agents,and novel doses and schedules of currently available agents.
Mortality in breast cancer has declined in the past decade, owing to advances in diagnosis, surgery, radiotherapy, and systemic treatments. Adjuvant chemotherapy has had a major effect on increasing survival in women with locoregional breast cancer. Like all treatments, adjuvant chemotherapy is a work in progress, and it has evolved from single oral agents to complex multidrug regimens. The choice of regimens is not without controversy, however, and several have been shown to be more effective than others, especially in patients who are at high risk for recurrence. The taxanes paclitaxel and docetaxel (Taxotere) have been shown to be effective in the adjuvant setting, and they have also been shown to improve the outcomes in node-positive disease. Both disease-free and overall survival are greater with doxorubicin, paclitaxel, and cyclophosphamide given in a dose-dense, every-2-week schedule with growth factor support than with the same agents given in an every-3-week schedule. Disease-free and overall survival in patients with node-positive disease are greater with docetaxel, doxorubicin (Adriamycin), and cyclophosphamide (TAC) than with fluorouracil, doxorubicin, and cyclophosphamide (FAC). Febrile neutropenia is common with the TAC regimen, but it can be minimized with growth factor support. Based on these findings, dose-dense therapy and TAC are the current adjuvant treatments of choice in patients with node-positive disease; other, less-intense regimens may be appropriate in patients with lower-risk disease. Ongoing trials are investigating the efficacy of commonly used regimens, new chemotherapeutic and biologic agents, and novel doses and schedules of currently available agents.
It has been estimated that close to 213,000 persons will be diagnosed with breast cancer in the United States in 2005.[1] Most of these patients will present with locoregional breast cancer, which relapses despite appropriate initial treatment. Mortality from breast cancer has decreased substantially, especially in patients with localized disease.[2] Both endocrine therapy and chemotherapy have led to substantial increases in survival among women with early-stage breast cancer.[3] Adjuvant endocrine therapy, which remains an essential component of treatment for patients with hormone receptor-positive disease, is not discussed in this article.[4,5] Adjuvant chemotherapy is a work in progress: It started as single-agent treatment,[6] was followed in the 1970s by combination chemotherapy with CMF (cyclophosphamide, methotrexate, and fluorouracil [5-FU]),[7] and now entails the use of various effective agents. Among the chemotherapy agents that are associated with greater survival are older drugs such as the alkylating agents (mainly cyclophosphamide), the antimetabolites (methotrexate and 5-FU), the anthracyclines (epirubicin [Ellence] and doxorubicin), and more recently, the taxanes (paclitaxel and docetaxel [Taxotere]). The 15-year update of findings from the Early Breast Cancer Trialists' Collaborative Group meta-analysis has shown that the risk of recurrence is 23.5% lower and the risk of mortality is 15.3% lower with several months of adjuvant combination chemotherapy than with no chemotherapy (both 2P < .001).[8] This article discusses the adjuvant regimens that are associated with the best outcomes, the selection of regimens on the basis of patient characteristics, and trials that have been completed or are in progress and are expected to substantially increase our knowledge in the next several years.
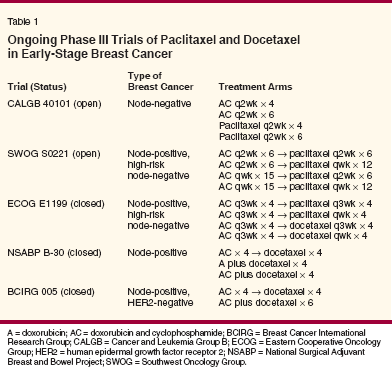
Strategies for the Use of Combination ChemotherapyChoice of Agents
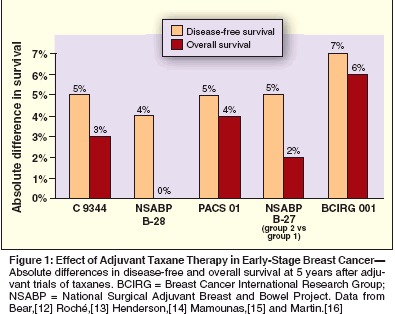
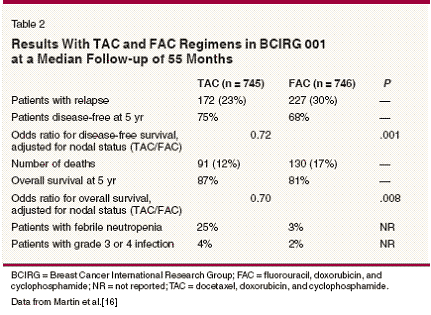
- Docetaxel-The combination of docetaxel, doxorubicin, and cyclophosphamide (TAC) has produced significantly higher complete and partial response rates than the combination of 5-FU, doxorubicin, and cyclophosphamide (FAC) in patients with metastatic breast cancer.[29] This regimen was tested in the adjuvant setting in the Breast Cancer International Research Group (BCIRG) 001 trial, which randomized 1,491 patients with node-positive breast cancer to six cycles of TAC (75/50/500 mg/m2) or FAC (500/50/500 mg/m2) every 3 weeks (Table 2).[16]
Interim analysis at a median follow- up of 55 months showed that 5-year disease-free survival was 7 percentage points greater and 5-year overall survival was 6 percentage points greater with TAC. The benefits of TAC on disease-free survival were not affected by hormone receptor status or HER2 status. Febrile neutropenia occurred in 24% of patients treated with TAC and in 2% of those treated with FAC.[30] Granulocyte colonystimulating factor (G-CSF, Neupogen) was used in all cycles after an occurrence of febrile neutropenia (secondary prophylaxis). An ongoing trial of TAC vs FAC in patients with nodenegative disease has confirmed the benefits of initiating G-CSF in the first cycle for minimizing neutropenic complications in patients treated with TAC.[31] NSABP B-27, a randomized trial of neoadjuvant chemotherapy in 2,411 women with operable breast cancer, compared three treatments: preoperative AC alone, preoperative AC followed by preoperative docetaxel, and preoperative AC followed by postoperative docetaxel.[12] The doses were the same in each arm-four cycles of AC (60/600 mg/m2) every 3 weeks and four cycles of docetaxel (100 mg/m2) every 3 weeks. The pathologic complete response rate with preoperative AC and docetaxel was twice that with preoperative AC alone (26.1% vs 13.7%; P < .001), and relapse-free survival at 5 years was also significantly higher (74% vs 69%; P = .03). Overall survival at 5 years was the same in all three arms. The concurrent use of tamoxifen and chemotherapy may have affected the outcomes in this trial.
The PACS 01 trial compared three cycles of FEC (5-FU, epirubicin, and cyclophosphamide at 500/100/500 mg/m2) followed by three cycles of docetaxel (100 mg/m2) with six cycles of adjuvant FEC (500/100/500 mg/m2) in 1,999 patients with node-positive operable breast cancer. At 5 years, both disease-free survival (78.3% vs 73.2%; P = .04) and overall survival (90.7% vs 86.7%; P = .05) were significantly higher with FEC followed by docetaxel than with FEC alone.[13] Since both regimens were used with the same total number of cycles (avoiding a potentially confounding factor seen in the earlier randomized trials of paclitaxel, CALGB 9344, and NSABP B-28, in which paclitaxel recipients were given more cycles of chemotherapy), the results in this trial suggest that docetaxel plays a major role in improving treatment outcomes. Moreover, cardiotoxicity was lower in the FEC/docetaxel arm. Two other large randomized trials are further evaluating the dose and sequence of anthracycline-docetaxel combinations (see Table 1). In NSABP B-30, patients with node-positive disease have been randomized to four cycles of concurrent doxorubicin and docetaxel (50/75 mg/m2), four cycles of TAC (75/50/500 mg/m2), or four cycles of AC (60/600 mg/m2) followed by four cycles of docetaxel (100 mg/m2). All cycles are repeated at 3-week intervals. In BCIRG 005, patients are randomized to six cycles of TAC (75/50/500 mg/m2) or four cycles of AC (60/600 mg/m2) followed by four cycles of docetaxel (100 mg/m2). The results of these trials should shed light on the optimal dosing and scheduling of anthracycline-docetaxel combinations in patients with early-stage breast cancer. Increasing Dose Intensity
Strong evidence supports the concept that the dose intensity of the chemotherapy, or the amount of drug delivered per unit of time, correlates with outcomes in the adjuvant setting.[ 32-34] A 30-year follow-up of patients in an early trial of adjuvant CMF shows that the survival advantage was greatest in those who were treated with more than 85% of the planned dose intensity (overall survival = 40% vs 21%).[35] At least three randomized trials have investigated the relationship between dose intensity and outcomes with anthracycline-based regimens. CALGB 8541 compared three regimens of adjuvant CAF (cyclophosphamide, doxorubicin, and 5-FU) in 1,550 patients with node-positive breast cancer: four cycles of 600/60/600 mg/m2 every 4 weeks (high dose intensity), six cycles of 400/40/400 mg/m2 every 4 weeks (moderate dose intensity), and four cycles of 300/30/300 mg/m2 every 4 weeks (low dose intensity).[ 33] The rates of both disease-free survival (P < .001) and overall survival (P = .004) were greater with the high- and moderate-dose-intensity regimens than with the low-doseintensity regimen. In a later trial in patients with nodepositive disease (CALGB 9344), the outcomes with the dose of doxorubicin increased to 75 or 90 mg/m2 were no better than those with the 60-mg/m2 dose.[14] Bonneterre and colleagues showed that an FEC regimen with epirubicin, 100 mg/m2, was superior to FEC with epirubicin, 50 mg/m2.[34] The results of these trials suggest that the effect of doxorubicin is maximal at a dose of about 50 to 60 mg/m2 and that the effect of epirubicin is maximal at a dose of about 100 mg/m2. More-intensive schedules of AC, tested in several large NSABP randomized trials, have proved to be no more efficacious as a standard schedule but much more toxic.[36,37] Extremely high doses of chemotherapy with stem cell rescue have not been found to be superior to standard regimens in randomized trials. Increasing Dose Density
Dose intensification can be achieved not only by increasing the dose intensity (increasing the dose of the drug per cycle) but also by increasing the dose density (decreasing the interval between the cycles while keeping the dose of the drug the same).[38] Mathematic models of tumor growth predict that shortening the interval between chemotherapy treatments would minimize tumor regrowth between cycles[39] and also lessen the emergence of drug-resistant mutations.[38,40] Bonadonna and colleagues were among the first to test dose-dense adjuvant therapy. In a study in women with early-stage breast cancer involving four or more lymph nodes, they compared 4 cycles of doxorubicin followed by 8 cycles of CMF (sequential regimen) and 2 cycles of CMF alternated with 1 cycle of doxorubicin for a total of 12 cycles (alternating regimen).[41] Overall survival at 10 years was 58% in the sequential arm and 44% in the alternating arm (P = .002). Thus, a clear survival advantage was shown for the sequential arm, which used the same total doses, but each agent was given at a higher dose density than in the alternating arm. More recently, Hudis and colleagues showed the feasibility of sequential doxorubicin, paclitaxel, and cyclophosphamide (A→T→C) given every 2 weeks (dose-dense regimen) with G-CSF support in 42 women with node-positive breast cancer.[42] The majority of patients (69%) developed febrile neutropenia, and red blood cell transfusions were required in 67%, but a 78% disease-free survival at 48 months was promising. Designed to further explore dosedense regimens, CALGB 9741 was a 2 * 2 trial that compared concurrent AC followed by paclitaxel and sequential A→T→C, both given either every 2 weeks (dose dense) or every 3 weeks, in 2,005 women with nodepositive breast cancer (Table 3).[43] The doxorubicin, cyclophosphamide, and paclitaxel were given at the same doses (60/600/175 mg/m2) in both arms. Patients in the dose-dense arms were given G-CSF to allow neutrophil recovery before the next cycle. Sequence had no effect on survival, but rates of both diseasefree survival (P = .01) and overall survival (P = .01) were significantly higher at a median follow-up of 36 months in the dose-dense arms.
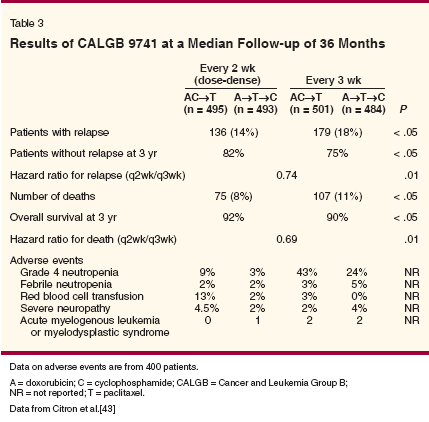
Furthermore, dose-dense therapy was not associated with greater toxicity, and the incidence of grade 4 neutropenia was greater in the every- 3-week sequential arm than in the two dose-dense arms (24%-43% for the every-3-week regimen vs 3%-9% for dose-dense therapy).[43] Platelet transfusions were not required in any patients, but red blood cell transfusions were required in 13% of patients in the concurrent dose-dense arm and in 3% of those in the every-3-week arm. An interim analysis of 89 patients (135 enrolled) treated with dose-dense therapy found that darbepoetin alfa (Aranesp), given at 200 ?g when the hemoglobin level dropped to less than 12 g/dL and administered thereafter according to a preplanned algorithm, eliminated the need for red blood cell transfusions.[44] The results of two European trials of dose-dense epirubicin-based regimens have been disappointing. Venturini and colleagues conducted a phase III trial in 1,214 patients with node-positive or high-risk node-negative operable breast cancer who were randomized to six cycles of FEC (600/60/600 mg/m2) at either 2- or 3-week intervals with G-CSF.[45] There were no significant differences in survival. In the neoadjuvant setting in patients with locally advanced breast cancer, survival with doseintense EC (120/830 mg/m2) given with G-CSF every 2 weeks for six cycles was no greater than that with FEC (500/60/75 mg/m2) given every 4 weeks for six cycles.[46] These data suggest that dose density may be more important with the taxanes than with other drugs. The benefits seen with dose density should not be extrapolated to untested regimens. Another strategy used with the taxanes is weekly regimens. A regimen of weekly paclitaxel was found to have greater efficacy than an every-3-week regimen, especially in metastatic disease.[ 47,48] The benefits of weekly paclitaxel in the neoadjuvant setting have also been shown: In a study in 258 patients with operable breast cancer, the response rate with preoperative weekly paclitaxel followed by FAC was double that with standard paclitaxel followed by FAC (29% vs 14%; P < .01).[47] The value of weekly paclitaxel as adjuvant therapy continues to be investigated and should be determined in ongoing trials. ECOG 1199, a randomized four-arm trial is comparing paclitaxel and docetaxel given either weekly or every 3 weeks after four cycles of AC. In another 2 * 2 trial design, SWOG S0221 will evaluate AC (weekly or every 2 weeks) followed by paclitaxel (weekly or every 2 weeks). The ongoing Intergroup S0221 trial is the only trial that is comparing weekly and every-2- week paclitaxel in the adjuvant setting. These data support the importance of delivering full chemotherapy doses on schedule. Dose reductions and delays, however, are common in clinical practice,[49,33,34] and when substantial, may affect outcomes. Clinicians should therefore avoid dose reductions whenever possible and should attempt to maintain the dose and schedule of the regimens that have been established in clinical trials.New Agents and Biologics
- Gemcitabine-Clinical trials in metastatic breast cancer serve as the cornerstone for the discovery of new effective agents worthy of testing in the adjuvant setting. Gemcitabine (Gemzar) has shown significant antitumor activity in the metastatic setting, with mild to moderate toxicity, both as a single agent and in combination with other agents, particularly paclitaxel.[50,51] A multinational randomized phase III trial in 529 patients with metastatic breast cancer who had been treated with anthracyclines found that the overall response rate was higher with gemcitabine plus paclitaxel than with paclitaxel alone (39.3% vs 25.6%; P < .001). The time to progression was also longer (5.4 vs 3.5 months, P = .002), and 1-year overall survival was higher for the combination regimen (70.7% vs 60.9%; P = .02). The combination regimen was associated with greater toxicity but better quality of life.[27]
A number of ongoing and planned trials are incorporating gemcitabine into adjuvant combination regimens in early-stage breast cancer.[50] A phase III trial in Britain (the tAnGo trial) will randomize patients to EC (90/600 mg/m2) followed by paclitaxel (175 mg/m2) alone or with gemcitabine (1,250 mg/m2 on days 1 and 8 of every 3-week cycle). In addition, NSABP B-38 is accruing patients to a trial in which they are randomized to TAC, dose-dense AC followed by paclitaxel (as in CALGB 9741), or dosedense AC followed by paclitaxel plus gemcitabine.
- Capecitabine-Capecitabine (Xeloda) is associated with minimal myelosuppression and alopecia, and because it is given orally, treatment at home is possible. Clinical trials are evaluating whether the clinical benefits with capecitabine in metastatic breast cancer are also seen in earlystage disease.[52] CALGB 49907 is a randomized comparison of singleagent capecitabine and standard CMF or AC in older patients. Quality of life and compliance will be evaluated in addition to safety and efficacy.
Numerous ongoing or planned trials will evaluate capecitabine in combination with taxanes, vinorelbine, or anthracyclines. The proposed British TACT2 trial is a 2 * 2 randomized adjuvant study in which 4,000 patients with mediumrisk breast cancer will be treated with standard or dose-dense epirubicin followed by CMF or capecitabine. A phase III adjuvant trial by US Oncology will compare AC followed by docetaxel plus capecitabine in patients with high-risk early-stage breast cancer. The same two regimens will be evaluated as preoperative therapy in another randomized trial, NSABP B27R.[52]
- Vinorelbine-The pilot phase II study GEPARTRIO randomized patients who had not responded to two cycles of neoadjuvant TAC to four additional cycles of preoperative TAC (n = 40) or vinorelbine plus capecitabine (n = 32). The pathologic complete response rate with vinorelbine plus capecitabine was less than that with TAC (3% vs 7%), but capecitabine plus vinorelbine was significantly less toxic.[53] An open-label randomized phase II trial (RMNHS-TOPIC2) is being conducted in Britain to evaluate three preoperative treatments in patients with stage I or II breast cancer: vinorelbine plus epirubicin, vinorelbine plus mitoxantrone (Novantrone), and AC.
- Trastuzumab-Trastuzumab (Herceptin), a monoclonal antibody that selectively binds to the extracellular domain of HER2, has shown clinical benefit as monotherapy[54] and in combination with chemotherapy[ 55] in patients with HER2-positive metastatic disease. Four large randomized trials that will enroll more than 12,000 women with HER2-positive breast cancer are investigating trastuzumab in the adjuvant setting. Clinical experience has shown that there can be a substantial risk of cardiotoxicity with trastuzumab when it is used concurrently with anthracyclines (doxorubicin cumulative doses of > 300 mg/m2).[56] Trials of trastuzumab in the adjuvant setting therefore have typically used sequential trastuzumab and anthracyclines, or nonanthracycline agents, and include thorough cardiac monitoring.
Just recently, the results of two major trials that randomized patients to trastuzumab or not have shown that the addition of trastuzumab to a variety of chemotherapeutic regimens resulted in a further lowering of relapse of about 50% after about 2 years of follow-up.[57,58] All patients who are HER2-positive should be considered for trastuzumab therapy after a discussion of the risks and benefits.
- Other Biologic Agents-Another novel agent that should be considered in the adjuvant setting is lapatinib, an oral inhibitor of both epidermal growth factor receptor and HER2 tyrosine kinases.[59] Lapatinib has shown efficacy and tolerability in patients with HER2-overexpressing advanced breast cancer that is refractory to trastuzumab-containing regimens.[ 60] Several phase II and III trials have been initiated to clarify the role of lapatinib used alone or with other agents in previously treated or untreated patients with advanced breast cancer.[59] The North Central Cancer Treatment Group (NCCTG), along with collaborators in the North American Breast Cancer Intergroup and International Cooperative groups, is developing a trial incorporating this agent as adjuvant therapy in patients with HER2-positive early-stage breast cancer.
Angiogenesis inhibitors have shown some efficacy in metastatic breast cancer. Bevacizumab (Avastin), a humanized monoclonal antibody to vascular endothelial growth factor, showed modest antitumor activity in patients with refractory metastatic breast cancer.[ 59,60] In a phase III trial, bevacizumab plus capecitabine improved overall reponse rate (19.8% vs 9.1%) but not disease-free survival compared with capecitabine alone in previously treated metastatic disease.[63] Evidence suggests that angiogenesis inhibitors may be most effective in the early stages of disease prior to metastasis,[ 64,65] and clinical trials of bevacizumab in combination with chemotherapy should be considered for early-stage breast cancer. Recently released data demonstrate significant improvement in disease-free survival when bevacizumab and weekly paclitaxel are administered concurrently compared to weekly paclitaxel alone. The addition of bevacizumab to paclitaxel increased the complete and partial response rate from 14% to 28% and the time to progression from 6 to 11 months compared to paclitaxel alone. There was also a significant prolongation of survival in this inital analysis.[66] Cyclooxygenase 2 is overexpressed in a substantial proportion of preinvasive and invasive breast cancers, and it is associated with aggressive or poor prognostic tumors.[67] Clinical trials of cyclooxygenase 2 inhibitors such as celecoxib (Celebrex) have been initiated in the metastatic and adjuvant settings, and their potential synergy with both nonsteroidal and steroidal aromatase inhibitors has been of particular interest.[67] Recent data that confirm the high incidence of cardiovascular events with these agents[68-70] have diminished interest in their continued evaluation, and their evaluation in the adjuvant setting has been stopped. Several hundred new targeted agents are in the drug-development pipeline, many with great promise in breast cancer. A challenge will be to design trials that quickly determine the most effective agents. Gene array technology and proteomics will likely play a crucial role in determining the targets for many of these agents, but clinical trials will be needed to define their benefits and toxicity.
Optimizing Adjuvant Chemotherapy Dose-dense chemotherapy, as in CALGB 9741, and TAC, as in BCIRG trial 001, have been associated with the best outcomes and with acceptable toxicity in patients with nodepositive breast cancer. Both of these regimens require G-CSF to minimize the occurrence of febrile neutropenia, and hemoglobin levels should be closely monitored in patients treated with dose-dense chemotherapy. The proactive use of erythropoietic agents in patients treated with dose-dense therapy who have hemoglobin levels less than 12 g/dL will likely eliminate the need for red blood cell transfusions. FEC 100 (ie, with epirubicin given at 100 mg/m2) is also a reasonable regimen in these high-risk patients. Dose-dense therapy or TAC should also be considered in patients with high-risk node-negative, estrogen receptor (ER)-negative and progesterone receptor (PR)-negative disease who are in otherwise good health and have a life expectancy of more than 10 years, but trials in such patients have not been conducted. The Early Breast Cancer Trialists' Collaborative Group found in their meta-analysis that the proportional reductions in relapse and mortality rates are similar in patients with node-negative and node-positive disease.[8] Thus, one would expect the same proportional reductions in relapse and mortality rates in patients with node-negative disease treated with dose-dense chemotherapy or TAC as those in patients with node-positive disease. The severe adverse events seen with dosedense therapy or TAC are not significantly different from those with less aggressive regimens, and patients with high-risk node-negative disease are suitable candidates for such treatment. Whether to use relatively aggressive regimens in patients with ER- or PR-positive high-risk node-negative disease is more controversial. Webbased calculators such as Adjuvant! (www.adjuvantonline.com) can help with this decision. Less aggressive regimens such as CMF and AC (for four cycles) might provide similar benefits as more-aggressive regimens in some of these patients and might be better in many patients, but trials to date have not had the statistical power to confirm whether that would be the case. The benefit of any chemotherapy regimen, regardless of its intensity, in patients with low-risk node-negative ER- or PR-positive breast cancer is less than that observed in patients with ER- and PR-negative disease. The potential benefits of chemotherapy in such patients should be estimated by using the calculators mentioned above, and they might also benefit from genetic analysis to determine their risk of recurrence with tamoxifen therapy.[ 70] Endocrine therapy is the mainstay of treatment in these patients, regardless of their age. Moreover, it is becoming clear that the benefits of endocrine therapy are related to the number of ER-positive cells-the more positive cells, the more effective the treatment.[71] Few data describe the risks and benefits of adjuvant chemotherapy in older women with early-stage breast cancer. The tumors in more than 75% of women with breast cancer aged 65 years and older are likely to be hormone receptor- positive, and endocrine therapy will remain a part of the mainstay of treatment in most of these patients. Conclusions Adjuvant TAC or dose-dense (every- 2-week) AC→T with G-CSF support should be considered as a component of standard of care in patients with node-positive early-stage breast cancer. The results from ongoing and planned clinical trials (evaluating novel schedules, biologic predictors, and targeted therapies) will help optimize treatment, as the drugs, doses, schedules, and standard of care in early-stage breast cancer continue to evolve.
Disclosures:
Dr. Muss is a consultant for a Pfizer DSMB, has ownership interest in Amgen and Enzon; has received research grants from AstraZeneca, Aventis, Bristol-Myers Squibb, Merck, GlaxoSmithKline, Ortho Biotech/Tibotech, Aureon, Celgene, Coley, Genentech, Genetics Institute, Imclone, Ligand, Lilly, Novartis, Pfizer, Sandoz, and Schering; fellowship support from Ortho, Amgen, Sanofi-Aventis, and MGI; honoraria from Network Oncology Communication, Neil Love Communications, Medidigm, and American Pharmaceutical; and is on the board of directors and advisory committees of the American Society of Clinical Oncology.
References:
1. Jemal A, Murray T, Ward E, et al: Cancer statistics, 2005. CA Cancer J Clin 55:10-30, 2005.
2. American Cancer Society: Cancer Facts & Figures 2005. Atlanta, American Cancer Society, 2005.
3. Early Breast Cancer Trialists’ Collaborative Group: Polychemotherapy for early breast cancer: An overview of the randomised trials. Lancet 352:930-942, 1998.
4. Pritchard KI: The best use of adjuvant endocrine treatments. Breast 12:497-508, 2003.
5. Eneman JD, Wood ME, Muss HB: Selecting adjuvant endocrine therapy for breast cancer (see discussion). Oncology (Huntingt) 18:1733-1744, 2004.
6. Fisher B, Carbone P, Economou SG, et al: 1-Phenylalanine mustard (L-PAM) in the management of primary breast cancer: A report of early findings. N Engl J Med 292:117-122, 1975.
7. Bonadonna G, Brusamolino E, Valagussa P, et al: Combination chemotherapy as an adjuvant treatment in operable breast cancer. N Engl J Med 294:405-410, 1976.
8. Early Breast Cancer Trialists’ Collaborative Group: Multi-agent chemotherapy for early breast cancer. Cochrane Database Syst Rev. (1):CD000487, 2002.
9. Fisher B, Brown AM, Dimitrov NV, et al: Two months of doxorubicin-cyclophosphamide with and without interval reinduction therapy compared with 6 months of cyclophosphamide, methotrexate, and fluorouracil in positive-node breast cancer patients with tamoxifen-nonresponsive tumors: results from the National Surgical Adjuvant Breast and Bowel Project B-15. J Clin Oncol 8:1483-1496, 1990.
10. Levine MN, Pritchard KI, Bramwell VCH, et al: A randomized trial comparing CEF to CMF in premenopausal women with node positive breast cancer: Update of NCIC CTG MA.5 (abstract). Breast Cancer Res Treat 76(suppl 1):S33, 2002.
11. Poole CJ, Earl HM, Dunn JA, et al: NEAT (National Epirubicin Adjuvant Trial) and SCTBG BR9601 (Scottish Cancer Trials Breast Group) phase III adjuvant breast trials show a significant relapse-free and overall survival advantage for sequential ECMF (abstract 13). Proc Am Soc Clin Oncol 22:4, 2003.
12. Bear HD, Anderson S, Smith RE, et al: A randomized trial comparing preoperative (preop) doxorubicin/cyclophosphamide (AC) to preop AC followed by preop docetaxel (T) and to preop AC followed by postoperative (postop) T in patients (pts) with operable carcinoma of the breast: Results of NSABP B-27 (abstract 26). Breast Cancer Res Treat 88(suppl 1):S16, 2004.
13. Roché H, Fumoleau P, Speilmann M, et al: Five years analysis of the PACS 01 trial: 6 cycles of FEC100 vs 3 cycles of docetaxel (D) for the adjuvant treatment of node positive breast cancer (abstract 27). Breast Cancer Res Treat 88(suppl 1):S16, 2004.
14. Henderson IC, Berry DA, Demetri GD, et al: Improved outcomes from adding sequential paclitaxel but not from escalating doxorubicin dose in an adjuvant chemotherapy regimen for patients with node-positive primary breast cancer. J Clin Oncol 21:976-983, 2003.
15. Mamounas E, Bryant J, Lembersky BC, et al: Paclitaxel (T) following doxorubicin/cyclophosphamide (AC) as adjuvant chemotherapy for node-positive breast cancer: Results from NSABP B-28 (abstract 12). Proc Am Soc Clin Oncol 22:4, 2003.
16. Martin M, Pienkowski T, Mackey J, et al: TAC improves disease free survival and overall survival over FAC in node positive early breast cancer patients, BCIRG 001: 55 months followup. Presented at the 26th Annual San Antonio Breast Cancer Symposium; December 3-6, 2003; San Antonio, Texas.
17. Nabholtz JM, Vannetzel JM, Llory JF, et al: Advances in the use of taxanes in the adjuvant therapy of breast cancer. Clin Breast Cancer 4:187-192, 2003b.
18. Goble S, Bear HD: Emerging role of taxanes in adjuvant and neoadjuvant therapy for breast cancer: The potential and the questions. Surg Clin North Am 83:943-971, 2003.
19. Perez EA: Adjuvant therapy approaches to breast cancer: Should taxanes be incorporated? Curr Oncol Rep 5:66-71, 2003.
20. Nabholtz JM, Falkson C, Campos D, et al: Docetaxel and doxorubicin compared with doxorubicin and cyclophosphamide as first-line chemotherapy for metastatic breast cancer: Results of a randomized, multicenter, phase III trial (erratum in J Clin Oncol 21:2048, 2003). J Clin Oncol 21:968-975, 2003.
21. Jassem J, Pienkowski T, Pluzanska A, et al: Doxorubicin and paclitaxel versus fluorouracil, doxorubicin, and cyclophosphamide as first-line therapy for women with metastatic breast cancer: Final results of a randomized phase III multicenter trial. J Clin Oncol 19:1707- 1715, 2001.
22. Biganzoli L, Cufer T, Bruning P, et al: Doxorubicin and paclitaxel versus doxorubicin and cyclophosphamide as first-line chemotherapy in metastatic breast cancer: The European Organization for Research and Treatment of Cancer 10961 multicenter phase 3 trial. J Clin Oncol 20:3114-3121, 2002.
23. Gligorov J, Lotz JP: Preclinical pharmacology of the taxanes: Implications of the differences. Oncologist 9(suppl 2):3-8, 2004.
24. Gianni L, Munzone E, Capri G, et al: Paclitaxel by 3-hour infusion in combination with bolus doxorubicin in women with untreated metastatic breast cancer: High antitumor efficacy and cardiac effects in a dose-finding and sequence-finding study. J Clin Oncol 13:2688- 2699, 1995.
25. Gianni L, Vigano L, Locatelli A, et al: Human pharmacokinetic characterization and in vitro study of the interaction between doxorubicin and paclitaxel in patients with breast cancer. J Clin Oncol 15:1906-1915, 1997.
26. Venturini M, Lunardi G, Del Mastro L, et al: Sequence effect of epirubicin and paclitaxel treatment on pharmacokinetics and toxicity. J Clin Oncol 18:2116-2125, 2000.
27. Albain KS, Nag S, Calderillo-Ruiz G, et al. Global phase III study of gemcitabine plus paclitaxel vs paclitaxel as frontline therapy for metastatic breast cancer: First report of overall survival (abstract 510). Proc Am Soc Clin Oncol 23:5, 2004.
28. Loesch D, Greco FA, O’Shaughnessy J, et al: A randomized, multicenter phase III trial comparing regimens of doxorubicin + cyclophosphamide followed by paclitaxel or doxorubicin + paclitaxel followed by weekly paclitaxel as adjuvant therapy for patients with high risk breast cancer (abstract). Breast Cancer Res Treat 88:S16, 2004.
29. Martin M, Pienkowski T, Mackey J, et al: Adjuvant docetaxel for node-positive breast cancer. N Engl J Med 352:2302-2313, 2005.
30. Nabholtz J, Pienkowski T, Mackey J, et al: Phase III trial comparing TAC (docetaxel, doxorubicin, cyclophosphamide) with FAC (5- fluorouracil, doxorubicin, cyclophosphamide) in the adjuvant treatment of node positive breast cancer (BC) patients: Interim analysis of the BCIRG 001 study (abstract 141). Proc Am Soc Clin Oncol 21:36a, 2002.
31. Martin M, Lluch A, Segui MA, et al: Prophylactic growth factor (GF) support with adjuvant docetaxel, doxorubicin, and cyclophosphamide (TAC) for node-negative breast cancer (BC): An interim safety analysis of the GEICAM 9805 study (abstract 620). Proc Am Soc Clin Oncol 23:32, 2004.
32. Bonadonna G, Valagussa P: Dose-response effect of adjuvant chemotherapy in breast cancer. N Engl J Med 304:10-15, 1981.
33. Budman DR, Berry DA, Cirrincione CT, et al: Dose and dose intensity as determinants of outcome in the adjuvant treatment of breast cancer. The Cancer and Leukemia Group B. J Natl Cancer Inst 90:1205-1211, 1998.
34. French Adjuvant Study Group: Benefit of a high-dose epirubicin regimen in adjuvant chemotherapy for node-positive breast cancer patients with poor prognostic factors: 5-year follow-up results of French Adjuvant Study Group 05 randomized trial. J Clin Oncol 19:602- 611, 2001.
35. Bonadonna G, Moliterni A, Zambetti M, et al: 30 years’ follow up of randomised studies of adjuvant CMF in operable breast cancer: Cohort study. BMJ 330:217, 2005.
36. Fisher B, Anderson S, Wickerham DL, et al: Increased intensification and total dose of cyclophosphamide in a doxorubicin-cyclophosphamide regimen for the treatment of primary breast cancer: Findings from National Surgical Adjuvant Breast and Bowel Project B-22. J Clin Oncol 15:1858-1869, 1997.
37. Fisher B, Anderson S, DeCillis A, et al: Further evaluation of intensified and increased total dose of cyclophosphamide for the treatment of primary breast cancer: Findings from National Surgical Adjuvant Breast and Bowel Project B- 25. J Clin Oncol 17:3374-3388, 1999.
38. Norton L: Evolving concepts in the systemic drug therapy of breast cancer. Semin Oncol 24(4 suppl 10):S10-S13, 1997.
39. Norton L: Biology of residual breast cancer after therapy: A kinetic interpretation. Prog Clin Biol Res 354A:109-132, 1990.
40. Coldman AJ, Goldie JH: Impact of doseintense chemotherapy on the development of permanent drug resistance. Semin Oncol 14:29- 33, 1987.
41. Bonadonna G, Zambetti M, Valagussa P: Sequential or alternating doxorubicin and CMF regimens in breast cancer with more than three positive nodes: Ten-year results. JAMA 273:542- 547, 1995.
42. Hudis C, Seidman A, Baselga J, et al: Sequential dose-dense doxorubicin, paclitaxel, and cyclophosphamide for resectable high-risk breast cancer: Feasibility and efficacy. J Clin Oncol 17:93-100, 1999.
43. Citron ML, Berry DA, Cirrincione C, et al: Randomized trial of dose-dense versus conventionally scheduled and sequential versus concurrent combination chemotherapy as postoperative adjuvant treatment of node-positive primary breast cancer: First report of Intergroup trial C9741/Cancer and Leukemia Group B trial 9741 (erratum in J Clin Oncol 21:2226, 2003). J Clin Oncol 21:1431-1439, 2003.
44. Burstein HJ, Parker LM, Doherty J, et al: Use of the long-acting hematopoietic growth factors pegfilgrastim and darbepoetin alfa in support of dose-dense adjuvant chemotherapy (abstract). Breast Cancer Res Treat 88:S60, 2004.
45. Venturini M, Aitini E, Del Mastro L, et al: Phase III adjuvant trial comparing standard versus accelerated FEC regimen in early breast cancer patients. Results from GONO-MIG1 study. Presented at the 26th Annual San Antonio Breast Cancer Symposium; December 3-6, 2003; San Antonio, Texas.
46. Therasse P, Mauriac L, Welnicka- Jaskiewicz M, et al: Final results of a randomized phase III trial comparing cyclophosphamide, epirubicin, and fluorouracil with a doseintensified epirubicin and cyclophosphamide + filgrastim as neoadjuvant treatment in locally advanced breast cancer: An EORTC-NCICSAKK multicenter study. J Clin Oncol 21:843- 850, 2003.
47. Green MJ, Buzdar A, Smith T, et al: Weekly (wkly) paclitaxel (P) followed by FAC as primary systemic chemotherapy (PSC) of operable breast cancer improves pathologic complete remission (pCR) rates when compared to every 3-week (Q 3 wk) P therapy (tx) followed by FAC- final results of a prospective phase III randomized trial (abstract 135). Proc Am Soc Clin Oncol 21:35a, 2002.
48. Seidman AD, Berry D, Cirrincione C, et al: CALGB 9840: Phase III study of weekly paclitaxel via 1-hour infusions versus standard 3h infusion every third week in the treatment of metastatic breast cancer, with trastuzumab for HER2 positive MBC and randomized for T in HER2 normal MBC. Presented at the 40th Annual Meeting of the American Society of Clinical Oncology; June 5-8, 2004; New Orleans.
49. Lyman GH, Dale DC, Crawford J: Incidence and predictors of low dose-intensity in adjuvant breast cancer chemotherapy: A nationwide study of community practices. J Clin Oncol 21:4524-4531, 2003.
50. Mamounas EP, Geyer CE Jr, Swain SM: Rationale and clinical trial design for evaluating gemcitabine as neoadjuvant and adjuvant therapy for breast cancer. Clin Breast Cancer 4(suppl 3):S121-S126, 2004.
51. Perez EA: Gemcitabine and platinum combinations in patients with breast cancer previously treated with anthracyclines and/or taxanes. Clin Breast Cancer 4(suppl 3):S113- S116, 2004.
52. Fumoleau P, Cameron D: Future options with capecitabine (Xeloda) in (neo)adjuvant treatment of breast cancer. Semin Oncol 31(5 suppl 10):45-50, 2004.
53. von Minckwitz G, Blohmer JU, Raab G, et al: In vivo chemosensitivity-adapted preoperative chemotherapy in patients with early-stage breast cancer: the GEPARTRIO pilot study. Ann Oncol 16:56-63, 2005.
54. Vogel CL, Franco SX: Clinical experience with trastuzumab (Herceptin). Breast J 9:452-462, 2003.
55. Slamon DL, Leyland-Jones B, Shak S, et al: Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med 344:783-792, 2001.
56. Perez EA, Rodeheffer R: Clinical cardiac tolerability of trastuzumab. J Clin Oncol 22:322- 329, 2004.
57. Romond EH, Perez EA, Bryant J, et al: Trastuzumab plus adjuvant chemotherapy for operable HER2-positive breast cancer. N Engl J Med 353:1673-1684, 2005
58. Piccart-Gebhart MJ, Procter M, Leyland- Jones B, et al: Trastuzumab after adjuvant chemotherapy in HER2-positive breast cancer. N Engl J Med 353:1659-1672, 2005.
59. Burris HA III: Dual kinase inhibition in the treatment of breast cancer: Initial experiencewith the EGFR/ErB-2 inhibitor lapatinib. Oncologist 9(suppl 3):10-15, 2004.
60. Blackwell KL, Kaplan EH, Franco SX, et al: A phase II, open-label, multicenter study of GW572016 in patients with trastuzumabrefractory metastatic breast cancer (abstract 3006). J Clin Oncol 22:14S, 2004.
61. Cobleigh MA, Langmuir BK, Sledge GW, et al: A phase I/II dose-escalation trial of bevacizumab in previously treated metastatic breast cancer. Semin Oncol 30:117-124, 2003.
62. Burstein HJ, Parker LM. Savoie J, et al: Phase II trial of the anti-VEGF antibody bevacizumab (Avastin) in combination with vinorelbine or refractory advanced breast cancer (abstract). Breast Cancer Res Treat 76(suppl 1):S115, 2002.
63. Miller KD, Rugo HS, Cobleigh MA, et al: Phase III trial of capecitabine (Xeloda) plus bevacizumab (Avastin) versus capecitabine alone in women with metastatic breast cancer previously treated with an anthracycline and a taxane (abstract). Breast Cancer Res Treat 76:S36, 2002.
64. Li CY, Shan S, Huang Q, et al. Initial stages of tumor cell-induced angiogenesis: Evaluation via skin window chambers in rodent models. J Natl Cancer Inst 2000;92:143-147.
65. Harmey JH, Bouchier-Hayes D: Vascular endothelial growth factor (VEGF), a survival factor for tumour cells: Implications for anti-angiogenic therapy. Bioessays 24:280-283, 2002.
66. Miller KD, Wang M, Bralow J, et al: E2100: A randomized phase III trial of paclitaxel versus paclitaxel plus bevacizumab as first-line therapy for locally recurrent or metastatic breast cancer. Annual meeting of the American Society of Clinical Oncology. Late-breaking session. Presented May 16, 2005.
67. Arun B, Goss P: The role of COX-2 inhibition in breast cancer treatment and prevention. Semin Oncol 31(2 suppl 7):22-29, 2004.
68. Solomon SD, McMurray JJ, Pfeffer MA, et al: Cardiovascular risk associated with celecoxib in a clinical trial for colorectal adenoma prevention. N Engl J Med 352:1071-1080, 2005.
69. Nussmeier NA, Whelton AA, Brown MT, et al: Complications of the COX-2 inhibitors parecoxib and valdecoxib after cardiac surgery. N Engl J Med 352:1081-1091, 2005.
70. Bresalier RS, Sandler RS, Quan H, et al: Cardiovascular events associated with rofecoxib in a colorectal adenoma chemoprevention trial. N Engl J Med 352:1092-1102, 2005.
71. Paik S, Shak S, Tang G, et al: A multigene assay to predict recurrence of tamoxifen-treated, node-negative breast cancer. N Engl J Med 351:2817-2826, 2004.
72. Harvey JM, Clark GM, Osborne CK, et al: Estrogen receptor status by immunohistochemistry is superior to the ligand-binding assay for predicting response to adjuvant endocrine therapy in breast cancer. J Clin Oncol 17:1474-1481, 1999.