Optimizing Outcomes of Advanced Prostate Cancer: Drug Sequencing and Novel Therapeutic Approaches
We have entered a period of accelerated drug development and optimism in the care of advanced prostate cancer. The treatment paradigm for these patients is rapidly evolving, with future study needed to define the optimal sequencing and potential combinations of these new agents.
The rapid approval of several novel agents has given prostate cancer patients and their treating physicians many new and effective therapeutic options. Three new medical therapies were recently approved on the basis of prolonged overall survival in castration-resistant prostate cancer patients: sipuleucel-T (Provenge), cabazitaxel (Jevtana), and abiraterone acetate (Zytiga). Additionally, there are several other promising prostate cancer agents in late-stage development, including MDV3100, PROSTVAC-VF (Prostvac), orteronel (TAK-700), and radium-223 chloride (Alpharadin), each with a novel mechanism of action. Taken together, we have entered a period of accelerated drug development and optimism in the care of advanced prostate cancer. The treatment paradigm for these patients is rapidly evolving, with future study needed to define the optimal sequencing and potential combinations of these new agents.
Introduction
Last year marked the start of an accelerated period of drug development for advanced prostate cancer. While gonadotropin-releasing hormone (GnRH) agonists and anti-androgens were approved in the 1980s, docetaxel, approved for prostate cancer in 2004, had been the only modern chemotherapy agent to demonstrate an overall survival advantage for men with castration-resistant prostate cancer (CRPC). Three new medical therapies were recently approved on the basis of prolonged overall survival in CRPC patients: sipuleucel-T (Provenge), cabazitaxel (Jevtana), and abiraterone acetate (Zytiga). Tables 1 and 2 provide further information about these agents; Table 2 also compares them with docetaxel and highlights their efficacy, safety, and costs. In addition, a novel new bone-targeting monoclonal antibody, denosumab (Xgeva), and an LHRH antagonist, degarelix (Firmagon), have been introduced into clinical practice.[1]
With the rapid introduction of these agents, questions arise about their optimal sequencing, in the context of our existing therapies. There are also many other novel agents currently under active development for prostate cancer, including a significant number in late-stage, phase III clinical trials for prostate cancer.
Sipuleucel-T
Sipuleucel-T was approved by the FDA in April 2010 for use in men with metastatic CRPC that is asymptomatic or minimally symptomatic (Table 1). It represents a first-in-class agent, classified as an autologous cellular immunotherapy. The manufacturing process for sipuleucel-T is unique and integral. Patients initially undergo collection of peripheral-blood mononuclear cells via leukapheresis, for enrichment for antigen-presenting cells. The patient’s blood is then delivered to a central manufacturing site, where it is processed with the recombinant fusion protein PA2024 (ProACT), which contains both antigenic (prostate acid phosphatase) and stimulatory (granulocyte-macrophage colony-stimulating factor) elements. This autologous, ex vivo loaded product is then shipped back to the patient and infused approximately 3 days after collection. This process takes place three times over a period of approximately 4 weeks.[2]
Sipuleucel-T was initially tested in a small study of 127 patients with symptomatic CRPC.[3] Subjects were randomized in 2:1 fashion to sipuleucel-T vs placebo. The primary endpoint was time to progression (TTP), with a secondary endpoint of overall survival; crossover to active therapy in those initially randomized to placebo was allowed. The median TTP was 11.7 weeks vs 10.0 weeks, favoring sipuleucel-T, but this was not statistically significant (P = .052). Median overall survival, however, was statistically significantly improved with sipuleucel-T, at 25.9 months vs 21.4 months with placebo (P = .01).
TABLE 1
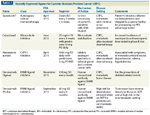
Recently Approved Agents for Castrate-Resistant Prostate Cancer (CRPC)
To follow up on these findings, a larger study was initiated.[2] This phase III trial included 512 subjects with metastatic CRPC who were randomized in 2:1 fashion to sipuleucel-T or placebo. Overall, therapy was well tolerated, with serious adverse events (grade 3-5) observed in 31.7% of sipuleucel-T-treated subjects, vs 35.1% of those in the placebo group. Adverse events that were more frequently observed in the sipuleucel-T group included chills (54.1%), pyrexia (29.3%), and headache (16.0%). The median overall survival time was 25.8 months with sipuleucel-T treatment vs 21.7 months in those randomized to placebo, remarkably similar to the survival results from the earlier randomized study. It is notable that 63.7% of those in the placebo arm did receive sipuleucel-T at some point in their subsequent treatments. The median survival of those in the placebo group who received delayed sipuleucel-T was 23.8 months vs 11.6 months in those who did not. The median time to objective disease progression and clinical disease progression was not different between the two treatment groups. One of the challenges with the use of sipuleucel-T is the lack of prostate-specific antigen (PSA) responses as a marker to evaluate activity.
There are few published data on activity of sipuleucel-T in the hormone-responsive or hormone-naive prostate cancer state. In a study by Beer et al, 176 patients with hormone-dependent prostate cancer and biochemical relapse after radical prostatectomy were randomized in a 2:1 ratio to 3 to 4 months of androgen-deprivation therapy (ADT), with or without sipuleucel-T.[4] The median time to biochemical failure was not statistically different in the sipuleucel or control groups (18.0 vs 15.4 months, respectively), although those with sipuleucel-T treatment did have a longer PSA doubling time (155 vs 105 days; P = .038). Based on the previous CRPC studies, overall survival may be more informative than TTP endpoints, and survival data for this study will require additional follow-up.
Cabazitaxel
Cabazitaxel was approved by the FDA in June 2010 for treatment of metastatic CRPC after treatment failure with docetaxel chemotherapy (Table 1). As discussed, docetaxel was approved for CRPC in 2004, and until 2010 it was the only agent to demonstrate a survival advantage in patients with CRPC.[5,6] Like docetaxel, cabazitaxel is a non–cross-resisted microtubule target agent, promoting tubulin assembly and thereby stabilizing the microtubule to the point of biological consequence.
TABLE 2
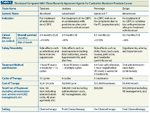
Docetaxel Compared With Three Recently Approved Agents for Castration-Resistant Prostate Cancer
In preclinical studies, cabazitaxel showed activity in a variety of chemotherapy-resistant cell lines and was more active than docetaxel in several models.[7] Phase I testing of cabazitaxel was reported in 2009, with dose-limiting neutropenia, but little neurotoxicity was observed.[8]
In phase III testing, 775 men with metastatic CRPC who had received previous docetaxel chemotherapy were randomized to prednisone with either cabazitaxel (at 25 mg/m2) or mitoxantrone (at 12 mg/m2), each given every 3 weeks.[9] The overall survival was 15.1 months with cabazitaxel, vs 12.7 months with mitoxantrone (P < .0001). The most common toxicities in the cabazitaxel arm were related to bone-marrow suppression, with grade 3 or higher neutropenia in 82% of the cabazitaxel-treated patients vs 58% of the mitoxantrone-treated patients. Additionally, febrile neutropenia was seen in 8% of those in the cabazitaxel arm compared with only 1% of the mitoxantrone arm. Diarrhea was also more common with cabazitaxel therapy, seen in 47% of patients, with 6% experiencing grade 3 or higher diarrhea.
Abiraterone acetate
Abiraterone acetate was approved by the FDA in April 2011 for the treatment of men with metastatic CRPC following docetaxel chemotherapy (Table 1). It is an oral inhibitor of CYP17, a key driver of testosterone production. While GnRH agonist/antagonist therapy reduces systemic testosterone production by 90% to 95% (via testicular suppression), the adrenal glands and some prostate cells themselves continue to synthesize testosterone despite GnRH agonist/antagonist treatment. Abiraterone acetate therapy directly suppresses this extragonadal testosterone production, yielding systemic testosterone levels approaching 0 ng/dL.[10,11]
Early phase I and II studies established activity of abiraterone acetate in CRPC. The initial phase I study of abiraterone acetate in CRPC escalated the dose from 250 to 2000 mg daily, with a recommended phase II dose of 1000 mg daily.[10] While generally well tolerated, increased levels of upstream steroids, including adrenocorticotropic hormone, were observed and side effects associated with mineralocorticoid excess were noted. In subsequent studies, dexamethasone, and later prednisone at 5 mg twice daily, was given with abiraterone to partially abrogate the accumulation of excess mineralocorticoid.[12] Across several of these studies, the rate of PSA responses (reductions of ≥ 50%) ranged from 36% to 67%.[10-14] Notably, subjects with previous ketoconazole treatment (given the similarities of ketoconazole’s adrenal-suppressive action) generally had a lower PSA response rate.[14]
A phase III study of abiraterone randomized 1195 subjects with metastatic CRPC and progression after docetaxel chemotherapy.[15] Participants received prednisone with or without 1000 mg of abiraterone acetate daily in a 2:1 ratio. Abiraterone acetate was well tolerated overall, although events associated with mineralocorticoid excess including fluid retention/edema (31%), hypokalemia (17%), and hypertension (10%) were observed more frequently in the abiraterone group. The median overall survival was 14.8 months with abiraterone acetate treatment, compared with 10.9 months in the placebo arm (P < .001). Secondary endpoints of PSA progression (10.2 months vs 6.6 months, respectively), progression-free survival (PFS) (5.6 months vs 3.6 months, respectively), and PSA response rate (29% vs 6%, respectively), all favored abiraterone acetate therapy.
Denosumab
Denosumab (Xgeva) was approved by the FDA in November 2010 for prevention of skeletal-related events (SREs) in patients with bone metastases from solid tumors (Table 1). A dynamic bone environment exists in normal bone, with a balance between bone production via osteoblasts and bone resorption via osteoclasts. SREs are common in prostate cancer due to dysregulation of bone formation/resorption, bone metastases themselves, and loss of bone mineral density associated with ADT. Receptor activator of nuclear factor kappa-B (RANK) ligand is critical to osteoclast formation and survival. Denosumab is a human monoclonal antibody against RANK ligand and it inhibits bone-resorption activity of osteoclasts.
In a phase III study of bone metastases and CRPC, 1904 men were randomized to denosumab (at 120 mg SQ) or zoledronic acid (Zometa; at 4 mg IV) every 4 weeks.[16] Median time to observance of the first SREs was 20.7 vs 17.1 months, favoring denosumab (P = .008). No overall survival difference was seen. Hypo-calcemia (P < .0001) and osteonecrosis of the jaw (P = .09) were noted more frequently in the denosumab group.
In addition to its initial label indication, denosumab was approved (under the trade name Prolia) in September 2011 to increase bone mass in patients who are at high risk of fracture and also are receiving ADT for nonmetastatic prostate cancer. In contrast to the dosing for metastatic CRPC, denosumab is given as a subcutaneous injection (Prolia, at 60 mg) every 6 months in the nonmetastatic setting. In the phase III study leading to this indication, high-risk prostate cancer subjects treated with ADT who were without bone metastases were randomized to denosumab or placebo, with 734 men in each group.[17] High-risk was defined as being 70 years of age or older, having low bone mineral density with a T score of < − 1.0, or having a history of an osteoporotic fracture. After 2 years on the study, the bone mineral density of the lumbar spine in the denosumab-treated group increased by 5.6%, while it had decreased 1.0 % in the placebo group (P < .001). Those treated with denosumab also had fewer new vertebral fractures at 36 months (1.5% vs 3.9%, P = .006).
A recently completed clinical trial in high-risk prostate cancer patients after local therapy revealed that denosumab is capable of delaying the development of bone metastasis. This study enrolled 1432 subjects with nonmetastatic CRPC and randomized them to denosumab (120 mg every 4 weeks) or placebo. The time to first metastasis was delayed by approximately 4 months in the denosumab-treated group.[18]
Sequencing of Medical Therapy in Advanced Prostate Cancer
FIGURE 1
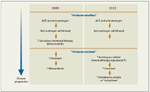
Sequencing of Medical Therapy in Prostate Cancer
The typical sequencing of medical therapy in advanced prostate cancer has been well defined in recent times, but the existing paradigm is being rewritten with the advent of these new therapies. The development and approval of these agents has generated an unprecedented excitement in prostate oncology.
Systemic therapy has generally been initiated with GnRH agonists/antagonists as monotherapy or in conjunction with an anti-androgen. With progressive disease, the anti-androgen was withdrawn, to assess for a therapeutic response, known as the anti-androgen withdrawal syndrome. At that point additional, secondary hormonal agents such as ketoconazole could be utilized, with modest data to support this approach. For those with aggressive or symptomatic disease and a good performance status, docetaxel chemotherapy was given (Figure 1).
This traditional schema for the medical management of prostate cancer has now changed. Rather than utilizing generally poorly studied secondary hormonal therapies for patients with CRPC, we now have sipuleucel-T available, which has a proven survival advantage in men with metastatic CRPC with minimal or no symptomatology. Additionally, for patients with CRPC after treatment with docetaxel, both abiraterone acetate and cabazitaxel are available. While there are no head-to-head trials to guide us, the side-effect profile of these agents, as outlined, is different and will in large measure dictate patient selection for these therapies. Thus, in the course of 2 years, we have four medical therapies rather than one for CRPC, approved based on a survival advantage.
FIGURE 2
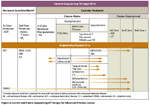
Current and Future Sequencing of Therapy for Advanced Prostate Cancer.
Emerging therapies
In additional to the agents highlighted above, there are other novel drugs in late-stage development, several of which are likely to change our treatment approach in prostate cancer in the coming years (Figure 2).
• MDV3100, a new-generation anti-androgen, is more potent and selective in targeting the androgen receptor (AR), impairing both nuclear translocation of the AR and its nuclear binding.[19] In a phase I/II study, 140 men with metastatic CRPC were treated with 30 mg to 600 mg of MDV3100, with PSA reductions of ≥ 50% observed in 56%.[20] Randomized phase III clinical trials of this agent are underway (clinicaltrials.gov identifier: NCT00974311 and NCT01212991). The AFFIRM (A study evaluating the eFFicacy and safety of Investigational dRug MDV3100 in men with advanced prostate cancer) post-docetaxel clinical trial was just reported to show a survival benefit for the agent. Initial reports of the post-docetaxel AFFIRM study from an interim analysis by the Independent Data Monitoring Committee (IDMC) indicate a 4.8-month survival advantage with use of MDV3100 vs placebo (13.6 vs 18.4 months; hazard ratio [HR] = 0.631).[21] The study was stopped on this basis, with subjects in the placebo arm to be offered MDV3100 therapy. Additionally, the IDMC indicated that the unblinded safety analysis yielded a favorable risk-to-benefit ratio for MDV3100.
• PROSTVAC-VF (Prostvac) is a PSA-targeted immunotherapy that utilizes a vaccinia vector for priming, followed by six fowlpox vector boosts.[22,23] A phase II study of PROSTVAC-VF with 125 patients randomized in 2:1 fashion to the vaccine vs control vectors has been reported.[24] There was no difference in the PFS, but the median overall survival was longer in the PROSTVAC-VF arm (25.1 months vs 16.6 months; P = .0061). This is in concordance with the sipuleucel-T phase III trials, in which immunotherapy improved overall survival but not PFS. A phase III study of PROSTVAC-VF is planned in men with metastatic CRPC who have minimal or no symptoms (clinicaltrials.gov identifier: NCT01322490).
• Orteronel (TAK-700) is a selective 17,20-lyase inhibitor, similar to abiraterone, which allows for a more targeted inhibition of the CYP17.[25] This selectivity may be associated with less mineralocorticoid excess, potentially leading to an improved side-effect profile. Phase III studies in CRPC are underway (clinicaltrials.gov identifier: NCT01193257 and NCT01193244).
• Alpharadin is an alpha-emitting radiopharmaceutical for the treatment of bone metastases (223RACl2).Compared with currently available radiopharmaceuticals such as strontium-89, Alpharadin utilizes alpha, not beta, particles, which have the advantage of more localized delivery of radiation, with the potential for less bone marrow toxicity.[26] Preliminary reports of a randomized, phase III trial (ALSYMPCA [ALpharadin in SYMptomatic Prostate Cancer]) have been reported at the 2011 European Cancer Organisation (ECCO)-European Society for Medical Oncology (ESMO) meetings in patients with CRPC and symptomatic bone involvement. The overall survival was improved, reported as 14.0 months with Alpharadin vs 11.2 months with placebo.[27]
REFERENCE GUIDE
Therapeutic Agents
Mentioned in This Article
Abiraterone acetate (Zytiga)
Anti-androgens
Cabazitaxel (Jevtana)
Degarelix (Firmagon)
Denosumab (Xgeva, Prolia)
Docetaxel
Gonadotropin-releasing hormone
agonists/antagonists
Granulocyte-macrophage colonystimulating
factor
Ipilimumab (Yervoy)
Ketoconazole
MDV3100
Mitoxantrone
Orteronel (TAK-700)
PA2024 antigen (ProACT)
Prednisone
Prostate acid phosphatase
PROSTVAC-VF (Prostvac)
Radium-223 chloride (Alpharadin)
Sipuleucel-T (Provenge)
Strontium-89
Tasquinimod
Zoledronic acid (Zometa)
Brand names are listed in parentheses only if a drug is not available generically and is marketed as no more than two trademarked or registered products. More familiar alternative generic designations may also be included parenthetically.
Adjuvant therapy
The addition of these novel agents to the therapeutic armamentarium for advanced prostate cancer raises exciting and challenging questions about a future role for medical therapy in the adjuvant setting. There is controversy about the use of adjuvant hormonal and/or chemotherapy in prostate cancer. This is largely due to a paucity of data, rather than negative data. The new agents highlighted here have not been evaluated in the adjuvant setting in a meaningful way. The largest modern effort to define the use of adjuvant chemotherapy/hormonal therapy in prostate cancer was Southwest Oncology Group (SWOG) trial 9921, in which men were randomized to 2 years of ADT, with or without mitoxantrone. Unfortunately, this study was closed before completing accrual due to the observation of leukemia in the mitoxantrone arm.[28] Notably, even though all subjects in the trial received ADT, the 5-year overall survival of the ADT-alone arm was recently reported as 95.9% and noted to be much better than predicted.[29] The 8-year median survival is currently estimated to be 88% (with a median follow-up of only 4.4 years), even though the trial initially assumed a median survival of 10 years in the control arm. While mitoxantrone was the only approved cytotoxic agent for advanced prostate cancer when SWOG 9921 was being designed, we now know that docetaxel is more efficacious than mitoxantrone, and that cabazitaxel is active in patients progressing after docetaxel. In this light, a large-scale adjuvant trial of cabazitaxel or docetaxel would be rational, as these are more active agents than have been available in the past. Additionally, the use of immunotherapy in the adjuvant setting is theoretically advantageous, utilizing this largely nontoxic therapy at the point of lowest disease burden for those at high risk. The challenges of completing a large, randomized trial to examine the role of medical therapy in this setting include the difficulty of defining a “high-risk” group, historically poor accrual to such trials, and the long follow-up period needed to assess the survival impact of any adjuvant therapy in prostate cancer. With the favorable results of ADT-alone in SWOG 9921, a very large study or a more narrowly defined “higher” risk group would need to be identified for future adjuvant studies.
Sequencing medical therapy in prostate cancer: a future outlook
TABLE 3

Future Considerations for Medical Therapy in Advanced Prostate Cancer
It will take many years to complete and fully report on the current studies of the agents in development reviewed here for prostate cancer. With four newly approved agents and several other promising drugs in late-stage development, future trials should be focused on defining the optimal application and timing of these therapies. Theoretically, active agents in prostate cancer with the least amount of toxicity should be used earliest in the disease process. For example, at the time of this writing, the FDA-approved indication for abiraterone acetate requires previous docetaxel therapy before its use. This is based on the patient population in the registration trial,[15] but it raises a clinical dilemma: for a patient with metastatic CRPC who is not a good candidate for docetaxel chemotherapy, abiraterone acetate use would be off-label, but would be a clinically rational and much less toxic approach than docetaxel. A trial of abiraterone acetate in this setting has been completed and results are forthcoming. Along this same line of reasoning, there are several unresolved questions regarding the optimal future use of these agents (Table 3), namely:
• Should active agents in CRPC with less toxicity, such as abiraterone acetate, be utilized before more toxic agents, such as docetaxel?
• Is cabazitaxel more efficacious than docetaxel as first-line cytotoxic therapy in CRPC?
• Could the early or adjuvant use of immunotherapy, which is currently approved for later-stage disease, improve long-term outcomes or even cure micrometastatic disease?
• Would monotherapy with a new-generation antiandrogen such as MDV3100 be as effective as traditional ADT for front-line hormonal therapy, and would such anti-androgen monotherapy be associated with an improved side-effect profile?
• Can any of these new agents (eg, sipuleucel-T with PROSTVAC-VF or abiraterone with MDV3100) be combined for greater efficacy with acceptable toxicity?
• What are the financial implications of all these new agents in the care of men with CRPC? (Table 2 outlines the agents and costs, which do not include the costs of drug administration, laboratory analysis, and supportive care.)
Summary
In conclusion, the rapid approval of several novel agents has given prostate cancer patients and their treating physicians many new and effective therapeutic options. This is a very exciting era in the management of CRPC. These newly approved agents and those in the pipeline present the real possibility of prolonging survival and the hope of turning the disease into a chronic condition. However, the optimal timing and the potential to combine these new agents with other medical therapies is not well defined. Over the last several years, the prostate cancer community has demonstrated the ability to successfully design and complete important trials, redefining the treatment of advanced prostate cancer. Moving forward, we must focus on defining the optimal use of these agents and supporting the clinical trials needed to accomplish this task.
Financial Disclosure: Dr. Crawford serves on the advisory board, and his wife is an employee, of Ferring. Dr. Crawford also serves on the advisory boards of sanofi- aventis, Janssen, Amgen and Dendreon. Dr. Flaig serves as a consultant to sanofi-aventis and has received an honorarium from Amgen.
References:
References
1. National Comprehensive Cancer Network: NCCN Guidelines. Version 4.2011. Prostate Cancer. Available at http://www.nccn.org/index.asp.
2. Kantoff PW, Higano CS, Shore ND, et al. Sipuleucel-T immunotherapy for castration-resistant prostate cancer. N Engl J Med. 2010;363:411-22.
3. Small EJ, Schellhammer PF, Higano CS, et al. Placebo-controlled phase III trial of immunologic therapy with sipuleucel-T (APC8015) in patients with metastatic, asymptomatic hormone refractory prostate cancer. J Clin Oncol. 2006;24:3089-94.
4. Beer TM, Bernstein GT, Corman JM, et al. Randomized trial of autologous cellular immunotherapy with sipuleucel-T in androgen-dependent prostate cancer. Clin Cancer Res. 2011;17:4558-67.
5. Petrylak DP, Tangen CM, Hussain MH, et al. Docetaxel and estramustine compared with mitoxantrone and prednisone for advanced refractory prostate cancer. N Engl J Med. 2004;351:1513-20.
6. Tannock IF, de Wit R, Berry WR, et al. Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer. N Engl J Med. 2004;351:1502-12.
7. Bissery M, Bouchard H, Riou JF, et al. Preclinical evaluation of TXD25, a new taxoid (abstract 1364). Proc Am Assoc Cancer Res. 2000;41:214.
8. Mita AC, Denis LJ, Rowinsky EK, et al. Phase I and pharmacokinetic study of XRP6258 (RPR 116258A), a novel taxane, administered as a 1-hour infusion every 3 weeks in patients with advanced solid tumors. Clin Cancer Res. 2009;15:723-30.
9. de Bono JS, Oudard S, Ozguroglu M, et al. Prednisone plus cabazitaxel or mitoxantrone for metastatic castration-resistant prostate cancer progressing after docetaxel treatment: a randomised open-label trial. Lancet. 2010;376:1147-54.
10. Attard G, Reid AH, Yap TA, et al. Phase I clinical trial of a selective inhibitor of CYP17, abiraterone acetate, confirms that castration-resistant prostate cancer commonly remains hormone driven. J Clin Oncol. 2008;26:4563-71.
11. Ryan CJ, Smith MR, Fong L, et al. Phase I clinical trial of the CYP17 inhibitor abiraterone acetate demonstrating clinical activity in patients with castration-resistant prostate cancer who received prior ketoconazole therapy. J Clin Oncol. 2010;28:1481-8.
12. Attard G, Reid AH, A’Hern R, et al. Selective inhibition of CYP17 with abiraterone acetate is highly active in the treatment of castration-resistant prostate cancer. J Clin Oncol. 2009;27:3742-8.
13. Reid AH, Attard G, Danila DC, et al. Significant and sustained antitumor activity in post-docetaxel, castration-resistant prostate cancer with the CYP17 inhibitor abiraterone acetate. J Clin Oncol. 2010;28:1489-95.
14. Danila DC, Morris MJ, de Bono JS, et al. Phase II multicenter study of abiraterone acetate plus prednisone therapy in patients with docetaxel-treated castration-resistant prostate cancer. J Clin Oncol. 2010;28:1496-501.
15. de Bono JS, Logothetis CJ, Molina A, et al. Abiraterone and increased survival in metastatic prostate cancer. N Engl J Med. 2011;364:1995-2005.
16. Fizazi K, Carducci M, Smith M, et al. Denosumab versus zoledronic acid for treatment of bone metastases in men with castration-resistant prostate cancer: a randomised, double-blind study. Lancet. 2011;377: 813-22.
17. Smith MR, Egerdie B, Hernandez Toriz N, et al. Denosumab in men receiving androgen-deprivation therapy for prostate cancer. N Engl J Med. 2009;
361:745-55.
18. Smith MR, Saad F, Coleman R, et al. Denosumab and bone-metastasis-free survival in men with castration-resistant prostate cancer: results of a phase 3, randomised, placebo-controlled trial. Lancet. 2011 Nov 15. [Epub ahead of print]
19. Tran C, Ouk S, Clegg NJ, et al. Development of a second-generation antiandrogen for treatment of advanced prostate cancer. Science. 2009;324:787-90.
20. Scher HI, Beer TM, Higano CS, et al. Antitumour activity of MDV3100 in castration-resistant prostate cancer: a phase 1-2 study. Lancet. 2010;375:1437-46.
21. Medivation and Astellas Oncology: Medivation and Astellas Announce Positive Survival Data From Interim Analysis of Phase 3 AFFIRM Trial of MDV3100 in Men With Advanced Prostate Cancer [news release]. Available at: http://investors.medivation.com/releasedetail.cfm?ReleaseID=620500.
22. DiPaola RS, Plante M, Kaufman H, et al. A phase I trial of pox PSA vaccines (PROSTVAC-VF) with B7-1, ICAM-1, and LFA-3 co-stimulatory molecules (TRICOM) in patients with prostate cancer. J Transl Med. 2006;4:1.
23. Arlen PM, Skarupa L, Pazdur M, et al. Clinical safety of a viral vector based prostate cancer vaccine strategy. J Urol. 2007;178:1515-20.
24. Kantoff PW, Schuetz TJ, Blumenstein BA, et al. Overall survival analysis of a phase II randomized controlled trial of a poxviral-based PSA-targeted immunotherapy in metastatic castration-resistant prostate cancer. J Clin Oncol. 2010;28:1099-105.
25. Kaku T, Hitaka T, Ojida A, et al. Discovery of orteronel (TAK-700), a naphthylmethylimidazole derivative, as a highly selective 17,20-lyase inhibitor with potential utility in the treatment of prostate cancer. Bioorg Med Chem. 2011;19:6383-99.
26. Bruland OS, Nilsson S, Fisher DR, Larsen RH. High-linear energy transfer irradiation targeted to skeletal metastases by the alpha-emitter 223Ra: adjuvant or alternative to conventional modalities? Clin Cancer Res. 2006;12:6250s-7s.
27. Journal of the National Cancer Institute: 103 ESMO Parker Transcript [podcast]. Available at: http://www.oxfordjournals.org/our_journals/jnci/podcast/ transcript_interview_103-esmo.html.
28. Flaig TW, Tangen CM, Hussain MH, et al. Randomization reveals unexpected acute leukemias in Southwest Oncology Group prostate cancer trial. J Clin Oncol 2008;26:1532-6.
29. Dorff TB, Flaig TW, Tangen CM, et al. Adjuvant androgen deprivation for high-risk prostate cancer after radical prostatectomy: SWOG S9921 study. J Clin Oncol. 2011;29:2040-5.