Nodal Marginal Zone B-Cell Lymphoma: A Diagnostic and Therapeutic Dilemma
The aim of this review is twofold: to summarize descriptions of the clinical presentation provided in published series in order to help clinicians recognize and treat patients, and to discuss diagnostic difficulties faced by hematopathologists when dealing with these lesions and others in the differential diagnosis that must be distinguished from one another.
In the last lymphoma classifications, three types of marginal zone lymphoma (MZL) were delineated: extranodal mucosa-associated lymphatic tissue (MALT) lymphoma, splenic MZL, and nodal MZL (NMZL). While MALT lymphoma is already well characterized and has been extensively studied, the pathogenesis of the other two types, especially that of NMZL, remains incompletely understood. The tumor is rather uncommon, although it shares morphologic and immunophenotypic similarities with the other MZLs. Few series have been published, and the description is quite heterogeneous, reflecting the lack of consensus criteria for its diagnosis; the ability to develop such criteria is impeded by the absence of specific immunological or molecular abnormalities. The disease develops from peripheral (mostly cervical) and abdominal lymph nodes, with or without bone marrow and blood involvement. How to differentiate NMZL from lymphoplasmacytic lymphoma remains a key point of debate. NMZL also represents a therapeutic dilemma, given the absence of published large or prospective series. The 5-year overall survival as well as the failure-free survival of patients appear to be lower than those of patients with extranodal MZL. The aim of this review is twofold: to summarize descriptions of the clinical presentation provided in published series in order to help clinicians recognize and treat patients, and to discuss diagnostic difficulties faced by hematopathologists when dealing with these lesions and others in the differential diagnosis that must be distinguished from one another.
Introduction
Nodal marginal zone B-cell lymphoma (NMZL) is a primary nodal B-cell lymphoma that shares morphologic, immunophenotypic, and genetic characteristics with extranodal marginal zone lymphoma (MZL) and splenic MZL, but without those specific localizations at presentation. NMZL was first described as “nodal monocytoid B-cell lymphoma” in 1986 by Sheibani et al.[1] In 1987, Cousar et al described it as “parafollicular B-cell lymphoma.”[2]The relationship with marginal zone B cells was established by Piris et al in 1988.[3] Nodal monocytoid B-cell lymphoma was introduced in the Revised Kiel classification by Lennert and Fellerin 1990.[4] NMZL, either with or without monocytoid B cells, was considered as a provisional subtype in the Revised European-American Lymphoma Classification in 2001.[5] In 2008, NMZL was finally admitted as a distinct entity in the World Health Organization (WHO) classification.[6] However, few series have been published, and discrepancies remain about the morphologic, biologic, and clinical characteristics of this disease.[7-20] There are also disagreements regarding therapeutic recommendations. In addition, the recent update of the WHO Lymphoma Classification has included a pediatric variant of NMZL, which has some distinguishing morphologic and clinical features.
Frequency and Epidemiology
Compared to other lymphomas, NMZL is rare; of the cases analysed in an international study,[15] NMZL represented 1.5%, and in a single-center series,[21] NMZL represented 1.8%. Two-thirds of the cases of the Southwest Oncology Group study[22] were described as “composite lymphomas” with concomitant follicular lymphoma, which might include follicular lymphomas with marginal zone differentiation. Other series probably include cases corresponding to nodal spread of extranodal marginal zone lymphoma or cases disseminated at diagnosis, with peripheral lymph nodes associated and/or extranodal or splenic involvement. The association of hepatitis C virus (HCV) infection with NMZL has been reported for the most part only in the Italian and Spanish series and appears rather rare in other settings.[20]
Diagnosis
Clinical presentation
Given the recent identification of NMZL, few reports present detailed clinical and outcome data from affected patients. Only 10 series are available, and these have relatively small numbers of patients (Table). The median age is 50 to 64 years; the sex ratio differs from one series to the next. The disease is localized in peripheral lymph nodes, mostly cervical and inguinal, with frequent involvement of other thoracic or abdominal nodes. The clinical stage at diagnosis varies among the series, but the majority of patients usually present with advanced clinical stage III or IV disease. Only two series reported patients with stage I or II disease.[16,23] Bone marrow infiltration is observed in 19% to 62% of cases, and peripheral blood involvement is very rare. The presence of B symptoms is infrequent. Elevated levels of β2-microglobulin are found in one-third of patients. An M component is detected in 5% to 33% of cases. A few cases have been reported to be associated with HCV infection (HCV seroprevalence was reported in 24% of patients in a series from Italy, in 20% of patients from Spain, and in 5% from Korea), but no such cases have been seen in our experience in France. Cryoglobulins may be present when associated with HCV infection. In contrast to the other MZL entities, there is no history of autoimmune disease in most patients with NMZL (although autoimmune hemolytic anemia has been reported in a subset of patients). Nodal involvement of other MZLs must be strictly excluded for diagnosis. Therefore, a careful clinical history is important when evaluating these cases.
Morphology
FIGURE 1
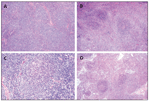
Immunoarchitectural Pattern of Nodal Marginal Zone Lymphomas
The morphologic features of NMZL are very heterogeneous (Figure 1), in terms of both architecture and cytology.[12,15,18] Different patterns of lymph node infiltration have been reported: marginal zone–like/perifollicular or “inverse follicular,” interfollicular, perisinusoidal, follicular via colonization of reactive follicles (less frequent than in MALT lymphomas), or diffuse. A combination of different patterns in a single case is a common finding. The morphologic evolution of the disease could be described as a perifollicular pattern characterized by enlargement of the marginal zone; followed by expansion into the interfollicular areas, follicular colonization, and formation of large nodules; and ending, in advanced cases, with total effacement of the lymph node architecture and a diffuse pattern. Residual atrophic follicles, which rarely are hyperplasic, are usually seen.
Unlike with splenic MZL, few data about bone marrow histology are available for NMZL.[18,24] A nodular and interstitial pattern has been reported. The sinusoidal localization that is more typical of splenic MZL is also frequently described in NMZL. As in splenic MZL, histology seems to be more sensitive than flow cytometry for the detection of bone marrow infiltration.[24]
FIGURE 2
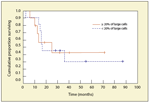
Outcomes in Patients With Nodal Marginal Zone Lymphoma According to the Proportion of Large Cells at Diagnosis
Several cell types may be encountered in varying proportions: small cells with irregular nuclei, clumped chromatin, and clear cytoplasm; cells resembling small lymphocytes; small cells with a plasmacytoid differentiation; plasma cells; and a variable content of medium to large cells that are centro-blast- or immunoblast-like. Follicular dendritic cells, usually arranged in a nodular meshwork or restricted to the perifollicular area (marginal zone pattern), are always present. “Monocytoid” B cells, which have more abundant and clear cytoplasm, are not usually predominant, and pure monocytoid B-cell lymphomas are less frequent than cases with plasmacytoid or plasmacytic differentiation. Unlike in MALT lymphoma and splenic MZL, the proportion of large cells is often relatively high (more than 20%), and the mitotic index is frequently elevated as well. These findings throw into question the classification of NMZL as a low-grade lymphoma. However, the component of large cells is always admixed with a small B-cell component without sheets of large cells, and the component of large cells sometimes colonizes the follicle. Those cases with a high proportion of large cells should not be considered as de novo diffuse large B-cell lymphoma because they retain the morphology previously described as characteristic of NMZL. In our experience, it seems that marginal zone B-cell lymphoma with a component composed purely of small cells is rarely seen at diagnosis; this is probably because biopsies are more often done for symptomatic patients and advanced-stage disease, which suggests a very indolent progression at the beginning of the disease. The high proportion of large cells and number of mitoses may explain the more aggressive clinical course reported in our previous series and those of others. However, in our experience, the number of large cells does not seem to influence the outcome in patients treated with polychemotherapy (Figure 2).
Immunophenotype
The phenotype, usually identical to that of extranodal MZL, is an important diagnostic feature that can help distinguish cases of NMZLs from other small B-cell lymphomas: typically the lymphoma cells are sIgM±D/G+, cIg±, CD19+, CD20+, CD79a+, Oct2+, Pax5+, CD5-, CD10-, CD23-, BCL2+, CyclinD1-. Few cases have been reported with expression of CD5 and/or CD23. CD43 is reported in up to 50% of cases. The expression of IgD has been reported by Campo,[14] who described a “splenic type” and a “MALT type” of NMZL. The splenic type (in the absence of splenic involvement) shows a nodular pattern with IgD positivity. In contrast, the MALT type shows a perivascular/perisinusoidal and parafollicular pattern, without expression of IgD. However, this subtyping is still debatable, and IgD expression has not been confirmed in a recent series of 51 cases.[25] The plasmacytic differentiation is usually associated with the expression of CD38, CD138, and MUM1.[16] In the largest series, the CD138 expression reflected an increase in plasma cell numbers in about half of the cases (24 of 51). In cases with follicle colonization, the benign reactive follicle center cells express CD10 and Bcl-6 and are negative for Bcl-2 and MUM1. In contrast, the colonizing MZL cells express Bcl-2 and often MUM1 and are negative for Bcl-6 and CD10.[26] Partially colonized follicles have exhibited a “moth-eaten” appearance on CD10, Bcl-2, Bcl-6, and MUM1 immunohistochemistry. In most cases, Ki67 expression is much higher among the residual benign/reactive follicle center cells than in the lymphoma cells themselves.[26]
Cytogenetic and Molecular Features
Cytogenetics may help with recognition of the disease (mainly by ruling out other small B-cell lymphomas); however, data remain sparse in the literature,[23,27-29] and it has been difficult to establish a characteristic cytogenetic profile for NMZL. Clonal aberrations are found in the majority of cases, and the karyotype is most often complex. Recurrent clonal abnormalities also found in the other types of MZLs-including trisomy 3, trisomy 18, trisomy 7, trisomy 12, and del6q-may contribute to the diagnosis. The presence of trisomy 12 could possibly be more frequent in NMZL than in MALT lymphoma or splenic MZL. The translocations characteristic of MALT or the 7q deletions that are recurrent in splenic MZL have not been reported in NMZL.[30] Rare cases (less than 10%) with heterozygous deletions on the TP53 gene detected by fluorescence in situ hybridization (FISH) have been reported.[27,31]
A comparative genomic hybridization approach has been used to better characterize the recurrent chromosome abnormalities, which are especially important in distinguishing between MZLs and Waldenstrm macroglobulinemia[32]; this approach has shown that the majority of the recurrent chromosome abnormalities identified in Waldenstrm macroglobulinemia are shared with MZLs-eg, deletions of 6q23 and 13q14, and gains of 3q13-q28, 6p, and 18q. In contrast, gains of 4q and 8q have been recurrently identified in Waldenstrm macroglobulinemia but have not been described as being common abnormalities in MZLs.[32]
Recently, genomic DNA copy number analysis was performed in a very large series of MZL cases,[33] including cases of MALT lymphoma, splenic MZL, and NMZL. This study showed no major lesions that were specific for NMZL, but it underscored the lack of splenic MZL–related 7q losses, and revealed a profile for NMZL that is more similar to that of MALT lymphomas,[33] as was also observed in a very recent gene expression study.[34] Inactivation of the A20 gene (localized on 6q23) by either somatic mutation and/or deletion has been described in 33% of NMZL cases (3 out of 9 cases); this represents a common genetic aberration across all MZL subtypes, one that may contribute to lymphomagenesis via induction of constitutive nuclear factor kappa B (NF-κB) activation.[33,35]
A comparative expression-profiling study has also shown a set of markers to be differently expressed in NMZL compared with follicular lymphoma; these include myeloid cell nuclear differentiation antigen (MNDA),[36] a nuclear protein expressed by myeloid cells and a subset of B cells. MNDA is expressed in normal tissue by a subset of marginal zone B cells; it is especially expressed in the three types of lymphoma derived from the marginal zone but is rarely seen in follicular lymphoma, a characteristic of potential value in distinguishing between NMZL and follicular lymphoma. A very recent gene and miRNA expression profiling analysis has confirmed the differences between the signatures of NMZL and follicular lymphoma.[37] This analysis shows that the NMZL gene expression profile reproduces the signature of normal marginal zone and memory B cells; in contrast, that of follicular lymphoma showed enriched expression of germinal center–linked genes. The analysis proposes new markers that can be used to differentiate between NMZL and follicular lymphoma, including CHIT1, TGFβ1, TAC1, miR-221, and miR-223 as markers for NMZL; and miR-494 as a marker for follicular lymphoma.
Immunoglobulin heavy-chain (IGHV) gene mutational status has been investigated in a limited number of small series.[16,38-42] The majority of cases (87%) presented with somatic mutations of the IGHV genes, with a biased usage of IGHV4-34, or IGHV1-69 in cases associated with HCV infection, and evidence of antigen selection in most cases, but without ongoing mutations. VH1-69–encoded antibodies have been shown to be specific for the viral antigen E2. No difference in outcome between patients with these mutations and those without mutations has been described.
Differential Diagnosis
Differentiating between NMZL and other small B-cell lymphomas can be difficult, since the latter sometimes have a marginal zone pattern or contain monocytoid B cells. Diagnosis of NMZL implies the exclusion of lymph node involvement associated with extranodal or splenic marginal zone B-cell lymphoma. Distinguishing between NMZL and reactive conditions-including T-zone hyperplasia, marginal zone expansion, and monocytoid B cell proliferation-on morphologic grounds can sometimes be problematic; however, NMZL can be distinguished with immunohistochemistry and clonality studies. Although elimination of mantle cell lymphoma or chronic lymphocytic leukemia can sometimes be challenging on the basis of cytological analysis of peripheral blood smears, these entities can be eliminated on the basis of specific phenotype and the presence of characteristic chromosomal abnormalities.
One of the entities in the differential diagnosis that can pose particular difficulties is follicular lymphoma with marginal zone differentiation; distinguishing between this and NMZL with follicular colonization is especially challenging.[26] In many follicles in patients with NMZL, the colonization is often partial; follicles also contain a reactive germinal center component. Immunophenotypic features can help clarify the diagnosis in such cases. Germinal center–associated markers that highlight follicular lymphoma cells are typically absent in NMZL, but aberrant expression has been reported. In addition, a subset of follicular lymphoma may also lack germinal center–associated markers, which could make distinguishing these cases of follicular lymphoma from NMZL particularly difficult. It seems that in challenging cases, additional germinal center markers (BCL6, HGAL, and LMO2) might show more sensitivity and specificity for follicular lymphoma cells. Follicular lymphomas that do not express CD10, as is the case in most instances of high grade 3B disease, instead express MUM1; such cases lack the BCL2 gene translocation but present with BCL6 gene abnormalities. Cytogenetic studies (BCL2 and/or BCL6 rearrangement by FISH) then assume greater importance to the diagnosis.
Differentiating between lymphoplasmacytic lymphoma and NMZL is also challenging, given the overlapping features of these two entities. Lymphoplasmacytic lymphoma is described in the WHO classification[36] as a lymphoma occurring in adults in the second half of life, involving bone marrow and less frequently (in 15% to 30% of cases) lymph nodes, spleen, liver, and sometimes peripheral blood. It is often associated with an IgM paraprotein. Waldenstrm macroglobulinemia is defined as lymphoplasmacytic lymphoma with bone marrow involvement and an IgM monoclonal gammapathy. Normal lymph node architecture is usually preserved; however, there may be dilated sinuses and sometimes atrophic residual germinal centers. An increased number of mast cells and hemosiderin are frequent findings. Other features are possible, however, and several are shared with NMZL: a diffuse or vaguely nodular pattern, plasmacytic differentiation, absence of specific phenotype, trisomy 3, association with HCV infection. In addition, literature data concerning lymphoplasmacytic lymphoma are limited and controversial; therefore, the relationship between lymphoplasmacytic lymphoma and MZL needs to be clarified.[38] Trisomy 4 seems to be recurrent in Waldenstrm macroglobulinemia.[43] The genetic changes in NMZL seem to differ from those reported in lymphoplasmacytic lymphoma, further supporting the distinction between these two entities. Additional studies, including a better characterization of the immunoglobulin IGHV repertoire in both NMZL and lymphoplasmacytic lymphoma (with only 46 and 53 cases reported, respectively) may help differentiate between these entities in the future.
Because the proportion of large cells is often high, many cases of NMZL may sometimes be confused with diffuse large B-cell lymphomas. In summary, it is likely that NMZL is underrecognized in routine practice, with cases mistakenly identified as indolent or as aggressive lymphomas.
Cases with prominent atrophic germinal centers and hyaline vascular penetration may closely resemble hyaline vascular Castelman disease (HV-CD), and are thus likely to be misdiagnosed.[44] In contrast to HV-CD, B-cell lymphomas with HV-CD–like features are more likely to manifest clinically with systemic symptoms or generalized lymphadenopathy. Careful histopathologic examination, supported by immunohistochemical studies, flow cytometric immunophenotyping, and judicious use of cytogenetic and molecular analyses, allows identification of the masked neoplastic process.
Prognostic and Predictive Factors
FIGURE 3
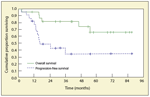
Progression-Free Survival and Overall Survival of 22 Patients With Nodal Marginal Zone Lymphoma
Outcomes in patients with NMZL are quite heterogeneous. Complete response to first-line treatment is seen in 50% to 60% of cases. In the International Lymphoma Study Group, 5-year failure-free survival and 5-year overall survival were only 28% and 56%, respectively.[45] Most studies have reported 5-year overall survivals in the range of 55% to 75%, with better outcomes in more recent series, possibly reflecting the increased use of rituximab (Rituxan). This trend toward a poor prognosis was also found in patients with a low or intermediate International Prognostic Index score, although the use of this index in NMZL is clearly exploratory. The 5-year overall survival is somewhat lower than those seen in follicular lymphoma and chronic lymphocytic leukemia, two of the most common low-grade B-cell neoplasms. In our series, time to progression was short-only 1.3 years-but median overall survival was close to 5 years, indicating that this disease may remain indolent for several years (Figure 3). Given the small numbers of cases included in different series, no specific prognostic factors have been reported for this entity. A poor performance status at diagnosis was the only clinical parameter that significantly influenced outcome. In the largest series,[46] the Follicular Lymphoma International Prognostic Index score also identified one-third of patients with a significantly shorter survival. Of note, a higher proportion of large cells in the diagnostic lymph node was not associated with a different outcome (see Figure 2). Patients who achieve a complete response to first-line treatment may also have a better prognosis. At the time of relapse, nodal sites are usually predominantly involved, although splenic or extranodal involvement may be encountered, in a manner reminiscent of the clinical features of the other MZL subtypes. However, histologic progression towards diffuse large-cell lymphomas appears to occur quite frequently, and there has been no evidence after the beginning of plateau survival curves to suggest that this disease is currently curable.
There are limited data concerning other prognostic markers in NMZL. In one study,[16] loss of survivin and loss of caspase 3 were associated with shorter failure-free survival, whereas a shorter overall survival was associated with increased age (older than 60 years) and overexpression of cyclin E. In another study,[47] patients lacking expression of MUM1 or expressing Ki67 in less than 5% of tumoral cells had a better prognosis.
Treatment
There is no standardized treatment for NMZL, but patients may be treated according to guidelines established for follicular lymphoma.[31,32] Patients with truly localized disease may be considered for localized radiation therapy, and good local tumor control is often achieved with this approach.[13-15] Treatment may be delayed in patients with a low tumor burden, or single-agent chemotherapy or immunotherapy can be proposed. In patients whose disease has more aggressive features, a standard immunochemotherapy regimen can be proposed, but a substantial proportion of patients do not achieve a complete response. A more dose-intensive strategy, eventually including autologous bone marrow transplantation, has sometimes been used in younger patients with a high number of large mitotic cells and adverse clinical prognostic factors.[33] Radiation therapy, using low doses of radiation, may also be considered as a palliative treatment in some cases.[34] However, none of these approaches has been prospectively tested. Therefore, no specific therapeutic approach can be recommended at this time, and clinicians will need to decide on the basis of the morphological and clinical characteristics of each patient. Monoclonal antibodies directed against the CD20 antigen have appeared in some reports to have some efficacy in this setting.[15]
Pediatric Nodal Marginal Zone Lymphoma
Pediatric NMZL is described as a separate variant of NMZL in the recent WHO classification of tumors of hematologic and lymphoid tissues.[6] It has a distinctive morphology and clinical presentation and stands out as an indolent disease with a remarkably better overall prognosis than classic NMZL. The median age of presentation is 16 years, and there is a striking male predominance (sex ratio, 20:1). In affected lymph nodes, the lymphoma cells have a predominantly interfollicular distribution, with marked expansion of the MZL. The cell component is similar to that of the classic form but with few large cells. A characteristic feature is follicular expansion with progressive transformation of germinal centers (“floral variant of NMZL”). Follicular colonization is also observed. Most of the cases present with an isolated nodal site of stage I disease and do well with a conservative approach following simple excision of the nodal mass. Few cases have been reported in adults, and it seems that correct identification is important in order to avoid unnecessary overtreatment of this indolent form. It is especially difficult to differentiate between pediatric NMZL and atypical marginal zone hyperplasia. Pediatric NMZL presents with a clonal rearrangement of IG genes and numerical genetic aberrations similar to those seen in adults. Therefore, the pathophysiology of pediatric NMZL remains poorly understood.
REFERENCE GUIDE
Therapeutic Agent
Mentioned in This Article
Rituximab (Rituxan)
Brand names are listed in parentheses only if a drug is not available generically and is marketed as no more than two trademarked or registered products. More familiar alternative generic designations may also be included parenthetically.
Future Directions
The morphologic and phenotypic description of NMZL is still incomplete in the literature. Cases that are truly borderline-eg, very similar to or perhaps overlapping with other types of lymphomas-appear to exist, and sorting through the differential diagnosis on the basis of morphology and phenotype may still sometimes be tenuous. Clinical and biologic studies of NMZL are hampered by the lack of specific diagnostic markers and the low reproducibility of this diagnosis. Furthermore, the existence of plasmacytic differentiation in NMZLs repeatedly raise the question of the genuine existence of lymphoplasmacytic lymphomas, and the literature is not clear yet on this matter. Overall, there is a strong need for a better individualization of these lymphomas and for an understanding of the mechanisms involved in their pathogenesis. Genomic and proteomic approaches are needed that can identify candidate markers specific to the diagnosis.
Financial Disclosure:The authors have no significant financial interest or other relationship with the manufacturers of any products or providers of any service mentioned in this article.
References:
REFERENCES
1. Sheibani K, Sohn CC, Burke JS, et al. Monocytoid B-cell lymphoma. A novel B-cell neoplasm. Am J Pathol. 1986;124:310-8.
2. Cousar JB, McGinn DL, Glick AD, et al. Report of an unusual lymphoma arising from parafollicular B-lymphocytes (PBLs) or so-called “monocytoid” lymphocytes. Am J Clin Pathol. 1987;87:121-8.
3. Piris MA, Rivas C, Morente M, et al. Monocytoid B-cell lymphoma, a tumour related to the marginal zone. Histopathology. 1988;12:383-92.
4. Lennert K, Feller AC. Histopathologie der non Hodgkin’s lymphoma (rach der acktulisierten Kiel-klassification). Berlin: Springer Verlag; 1990.
5. Jaffe ES, Harris NL, Stein H, Vardiman JW. Tumours of haematopoietic and lymphoid tissues. Lyon: 2001.
6. Swerdlow SH. WHO classification of tumours of haematopoietic and lymphoid tissues. 4th ed. Lyon: International Agency for Research on Cancer; 2008.
7. Ng CS, Chan JK. Monocytoid B-cell lymphoma. Hum Pathol. 1987;18:1069-71.
8. Cogliatti SB, Lennert K, Hansmann ML, Zwingers TL. Monocytoid B cell lymphoma: clinical and prognostic features of 21 patients. J Clin Pathol. 1990;43:619-25.
9. Ngan BY, Warnke RA, Wilson M, et al. Monocytoid B-cell lymphoma: a study of 36 cases. Hum Pathol. 1991;22:409-21.
10. Nizze H, Cogliatti SB, von Schilling C, et al. Monocytoid B-cell lymphoma: morphological variants and relationship to low-grade B-cell lymphoma of the mucosa-associated lymphoid tissue. Histopathology. 1991;18:403-14.
11. Ortiz-Hidalgo C, Wright DH. The morphological spectrum of monocytoid B-cell lymphoma and its relationship to lymphomas of mucosa-associated lymphoid tissue. Histopathology. 1992;21:555-61.
12. Nathwani BN, Drachenberg MR, Hernandez AM, et al. Nodal monocytoid B-cell lymphoma (nodal marginal-zone B-cell lymphoma). Semin Hematol. 1999;36:128-38.
13. Nathwani BN, Anderson JR, Armitage JO, et al. Marginal zone B-cell lymphoma: a clinical comparison of nodal and mucosa-associated lymphoid tissue types. Non-Hodgkin’s Lymphoma Classification Project. J Clin Oncol. 1999;17:2486-92.
14. Campo E, Miquel R, Krenacs L, et al. Primary nodal marginal zone lymphomas of splenic and MALT type. Am J Surg Pathol. 1999;23:59-68.
15. Berger F, Felman P, Thieblemont C, et al. Non-MALT marginal zone B-cell lymphomas: a description of clinical presentation and outcome in 124 patients. Blood. 2000;95:1950-6.
16. Camacho FI, Algara P, Mollejo M, et al. Nodal marginal zone lymphoma: a heterogeneous tumor: a comprehensive analysis of a series of 27 cases. Am J Surg Pathol. 2003;27:762-71.
17. Oh SY, Ryoo BY, Kim WS, et al. Nodal marginal zone B-cell lymphoma: analysis of 36 cases. Clinical presentation and treatment outcomes of nodal marginal zone B-cell lymphoma. Ann Hematol. 2006;85:781-6.
18. Traverse-Glehen A, Felman P, Callet-Bauchu E, et al. A clinicopathological study of nodal marginal zone B-cell lymphoma. A report on 21 cases. Histopathology. 2006;48:162-73.
19. Arcaini L, Paulli M, Boveri E, et al. Marginal zone-related neoplasms of splenic and nodal origin. Haematologica. 2003;88:80-93.
20. Arcaini L, Paulli M, Boveri E, et al. Splenic and nodal marginal zone lymphomas are indolent disorders at high hepatitis C virus seroprevalence with distinct presenting features but similar morphologic and phenotypic profiles. Cancer. 2004;100:107-15.
21. A clinical evaluation of the International Lymphoma Study Group classification of non-Hodgkin’s lymphoma. The Non-Hodgkin’s Lymphoma Classification Project. Blood. 1997;89:3909-18.
22. Fisher RI, Dahlberg S, Nathwani BN, et al. A clinical analysis of two indolent lymphoma entities: mantle cell lymphoma and marginal zone lymphoma (including the mucosa-associated lymphoid tissue and monocytoid B-cell subcategories): a Southwest Oncology Group study. Blood. 1995;85:1075-82.
23. Kojima M, Tsukamoto N, Miyazawa Y, et al. Nodal marginal zone B-cell lymphoma associated with Sjogren’s syndrome: a report of three cases. Leuk Lymphoma. 2007;48:1222-4.
24. Boveri E, Arcaini L, Merli M, et al. Bone marrow histology in marginal zone B-cell lymphomas: correlation with clinical parameters and flow cytometry in 120 patients. Ann Oncol. 2009;20:129-36.
25. Salama ME, Lossos IS, Warnke RA Natkunam Y. Immunoarchitectural patterns in nodal marginal zone B-cell lymphoma: a study of 51 cases. Am J Clin Pathol. 2009;132:39-49.
26. Naresh KN. Nodal marginal zone B-cell lymphoma with prominent follicular colonization-difficulties in diagnosis: a study of 15 cases. Histopathology. 2008;52:331-9.
27. Dierlamm J, Pittaluga S, Wlodarska I, et al. Marginal zone B-cell lymphomas of different sites share similar cytogenetic and morphologic features. Blood. 1996;87:299-307.
28. Banerjee SS, Harris M, Eyden BP, et al. Monocytoid B cell lymphoma. J Clin Pathol. 1991;44:39-44.
29. Slovak ML, Weiss LM, Nathwani BN, et al. Cytogenetic studies of composite lymphomas: monocytoid B-cell lymphoma and other B-cell non-Hodgkin’s lymphomas. Hum Pathol. 1993;24:1086-94.
30. Remstein ED, James CD, Kurtin PJ. Incidence and subtype specificity of API2-MALT1 fusion translocations in extranodal, nodal, and splenic marginal zone lymphomas. Am J Pathol. 2000;156:1183-8.
31. Callet-Bauchu E, Baseggio L, Felman P, et al. Cytogenetic analysis delineates a spectrum of chromosomal changes that can distinguish non-MALT marginal zone B-cell lymphomas among mature B-cell entities: a description of 103 cases. Leukemia. 2005;19:1818-23.
32. Braggio E, Keats JJ, Leleu X, et al. High-resolution genomic analysis in Waldenstrom’s macroglobulinemia identifies disease-specific and common abnormalities with marginal zone lymphomas. Clin Lymphoma Myeloma. 2009;9:39-42.
33. Rinaldi A, Mian M, Chigrinova E, et al. Genome-wide DNA profiling of marginal zone lymphomas identifies subtype-specific lesions with an impact on the clinical outcome. Blood. 117:1595-604.
34. Novak U, Basso K, Pasqualucci L, et al. Genomic analysis of non-splenic marginal zone lymphomas (MZL) indicates similarities between nodal and extranodal MZL and supports their derivation from memory B-cells. Br J Haematol. 2011;155:362-5.
35. Novak U, Rinaldi A, Kwee I, et al. The NF-ÎB negative regulator TNFAIP3 (A20) is commonly inactivated by somatic mutations and genomic deletions in marginal zone B-cell lymphomas. Blood. 2009;113:4918-21.
36. Kanellis G, Roncador G, Arribas A, et al. Identification of MNDA as a new marker for nodal marginal zone lymphoma. Leukemia. 2009;23:1847-57.
37. Arribas JA, Campos-Martin Y, Gómez-Abad C, et al. Nodal marginal zone lymphoma: gene expression and miRNA profiling identify diagnostic markers and potential therapeutic targtets. Blood. 2011 Nov 22. [Epub ahead of print]
38. Tierens A, Delabie J, Pittaluga S, et al. Mutation analysis of the rearranged immunoglobulin heavy chain genes of marginal zone cell lymphomas indicates an origin from different marginal zone B lymphocyte subsets. Blood. 1998;91:2381-6.
39. Conconi A, Bertoni F, Pedrinis E, et al. Nodal marginal zone B-cell lymphomas may arise from different subsets of marginal zone B lymphocytes. Blood. 2001;98:781-6.
40. Traverse-Glehen A, Davi F, Ben Simon E, et al. Analysis of VH genes in marginal zone lymphoma reveals marked heterogeneity between splenic and nodal tumors and suggests the existence of clonal selection. Haematologica. 2005;90:470-8.
41. Miranda RN, Cousar JB, Hammer RD, et al. Somatic mutation analysis of IgH variable regions reveals that tumor cells of most parafollicular (monocytoid) B-cell lymphoma, splenic marginal zone B-cell lymphoma, and some hairy cell leukemia are composed of memory B lymphocytes. Hum Pathol. 1999;30:306-12.
42. Kuppers R, Hajadi M, Plank L, et al. Molecular Ig gene analysis reveals that monocytoid B cell lymphoma is a malignancy of mature B cells carrying somatically mutated V region genes and suggests that rearrangement of the kappa-deleting element (resulting in deletion of the Ig kappa enhancers) abolishes somatic hypermutation in the human. Eur J Immunol. 1996;26:1794-800.
43. Terre C, Nguyen-Khac F, Barin C, et al. Trisomy 4, a new chromosomal abnormality in Waldenstrom’s macroglobulinemia: a study of 39 cases. Leukemia. 2006;20:1634-6.
44. Siddiqi IN, Brynes RK Wang E. B-cell lymphoma with hyaline vascular Castleman disease-like features: a clinicopathologic study. Am J Clin Pathol. 135:901-14.
45. Armitage JO, Weisenburger DD. New approach to classifying non-Hodgkin’s lymphomas: clinical features of the major histologic subtypes. Non-Hodgkin’s Lymphoma Classification Project. J Clin Oncol. 1998;16:2780-95.
46. Arcaini L, Paulli M, Burcheri S, et al. Primary nodal marginal zone B-cell lymphoma: clinical features and prognostic assessment of a rare disease. Br J Haematol. 2007;136:301-4.
47. Petit B, Chaury MP, Le Clorennec C, et al. Indolent lymphoplasmacytic and marginal zone B-cell lymphomas: absence of both IRF4 and Ki67 expression identifies a better prognosis subgroup. Haematologica. 2005;90:200-6.
Navigating AE Management for Cellular Therapy Across Hematologic Cancers
A panel of clinical pharmacists discussed strategies for mitigating toxicities across different multiple myeloma, lymphoma, and leukemia populations.