Ovarian Suppression/Ablation in Premenopausal ER-Positive Breast Cancer Patients
Developed over a century ago,[1] endocrine therapy remains the most effective and the most clearly targeted form of systemic therapy for breast cancer. Endocrine treatments work best in women whose tumors are positive for estrogen receptors (ER) and/or progesterone receptors (PR).
Endocrine therapy remains pivotal in the adjuvant therapy of premenopausal women with hormone receptor–positive breast cancer. Ovarian ablation, used alone, is effective in delaying recurrence and increasing survival in such women. When added to chemotherapy, it is less clear that this technique is effective, perhaps because of the endocrine ablative effect of chemotherapy. Adjuvant trials comparing ovarian ablation with or without tamoxifen to CMF-type chemotherapy (cyclophosphamide, methotrexate, fluorouracil) suggest that the endocrine therapy is equivalent to or better than this chemotherapy in women whose tumors express estrogen and/or progesterone receptors. Endocrine therapy with ovarian ablation, tamoxifen, or the combination is also useful in the metastatic setting in premenopausal women.
Developed over a century ago,[1] endocrine therapy remains the most effective and the most clearly targeted form of systemic therapy for breast cancer. Endocrine treatments work best in women whose tumors are positive for estrogen receptors (ER) and/or progesterone receptors (PR). As we search for newer targeted therapies that will shrink cancers effectively with few undesired side effects and carry out complex statistical analyses to identify predictive factors, we should keep in mind the first targeted cancer therapy-ovarian ablation for breast cancer-and the first predictive factor for treatment of any cancer-the estrogen receptor.[2]
Adjuvant treatment for premenopausal women is an important current focus in the setting of breast cancer. In Canada, 20% of newly diagnosed breast cancer cases occur in patients under 50 years of age, 4% are in women under 40, and 1% to 2% are in those under 35.[3] Of these, about half will have ER- and/or PR-positive disease.
Ovarian Ablation as Adjuvant Endocrine Therapy
or many years, adjuvant ovarian ablation was believed to be helpful in premenopausal women with breast cancer, but randomized trials were not done. A few small randomized trials were carried out in the 1960s and 1970s, but prior to the first Early Breast Cancer Trialists’ Collaborative Group (EBCTCG) Overview (aka the Oxford Overview) in 1984,[4] it was generally thought that these trials showed no benefit for ovarian ablation. This strategy therefore came to be considered an outmoded breast cancer therapy. When the meta-analytic techniques used in the EBCTCG Overview were applied to these small trials, however, it became apparent that ovarian ablation was effective.
Ovarian Ablation as Primary Adjuvant Systemic Therapy
EBCTCG Overview
The meta-analytic techniques used in the EBCTCG Overview have shown that ovarian ablation is associated with a relatively large positive effect on both disease-free survival (DFS) and overall survival (OS) in premenopausal women. In women with or without involved axillary lymph nodes,[4-6] the effects of ovarian ablation were significantly positive in the subgroup of trials in which women were randomized to receive ovarian ablation alone vs no systemic therapy. However, studies of ovarian ablation plus chemotherapy vs the same chemotherapy alone showed only a slight trend toward a positive effect.[4-6] The lack of a large and obvious benefit in women who also received chemotherapy may relate to the ovarian suppressive effect secondary to chemotherapy, which occurs in many premenopausal women. This effect may be less applicable in very young women, but the data-even in the EBCTCG Overview-are too scant to be sure.
The most recent EBCTCG Overview, carried out in September 2005–2006, included updated information on 12,000 women under age 50 in 16 trials of ovarian ablation/suppression vs no treatment of this sort. There were more women and more breast cancer deaths in trials of ovarian ablation in the presence of chemotherapy (more than 3,300 recurrences and more than 2,800 deaths) than in trials of ovarian ablation in the absence of chemotherapy (about 1,000 recurrences and 900 breast cancer deaths) (personal communication, R. Peto, 2006).
This updated analysis showed a clear difference between the trials of ovarian ablation vs no treatment in the absence of chemotherapy-in which a large and highly significant positive effect persisted with regard to DFS (52.2% vs 40.8% at 15 years [difference = 11.5%; standard error (SE) = 2.6])-and the trials of ovarian ablation plus chemotherapy vs the same chemotherapy, which showed no significant difference with regard to DFS (54.7% vs 55.3% [difference = 0.6%; SE = 2.9]). Breast cancer deaths were also reduced by about 5% after 10 years and by about 11% after 20 years in trials of ovarian ablation/suppression in the absence of chemotherapy, but by only 2% to 3% after 10 years in the presence of chemotherapy. Twenty-year data are not available for trials of ovarian ablation/suppression in the presence of chemotherapy.
LHRH Analog
The development of the luteinizing hormone-releasing hormone (LHRH) analogs has increased interest in the use of ovarian ablation in premenopausal women. In one fairly large phase III trial,[7] an LHRH analog was shown to be as effective as surgical ovarian ablation in the treatment of metastatic breast cancer. In addition, a meta-analysis of four small studies comparing tamoxifen plus an LHRH analog to an LHRH analog alone have suggested that the combination is superior in terms of disease progression and overall survival in the metastatic setting.[8] It has been suggested that this observation might extend into the adjuvant setting, where the combination of an LHRH analog and tamoxifen could prove superior to either alone.

Various LHRH analogs-in particular, the drug goserelin (Zoladex)-have now been tested in large trials of adjuvant therapy. The study designs are of two types: (1) those comparing goserelin, tamoxifen, or goserelin plus tamoxifen to chemotherapy in the premenopausal setting, and (2) those that add either goserelin, tamoxifen, or goserelin plus tamoxifen to chemotherapy in the same group of women.
In the most recent EBCTCG Overview (September 2006), five trials of the LHRH agonist goserelin vs no goserelin in 5,700 women were identified. As in 2000, data from only three of these trials in 4,200 women were available. These trials are included with those of ovarian ablation in the EBCTCG synopsis above. In the Eastern Cooperative Oncology Group (ECOG) E5188 trial,[9] all patients received CAF chemotherapy (cyclophosphamide, doxorubicin [Adriamyin], fluorouracil [5-FU]). In the Zoladex In Premenopausal Patients (ZIPP) trial, 1,173 of 2,710 women received chemotherapy prior to randomization to goserelin and/or tamoxifen.[10] In the French Fdration Nationale des Centres de Lutte contre le Cancer (FNCLCC) trial, all 16,212 women received chemotherapy before being randomized to goserelin or control.[11]
ZIPP Trial
he ZIPP trial has been independently reported and now fully published.[10] Its design was that of a large 2×2 factorial study in which premenopausal women with early-stage disease were randomized, after primary surgery, to (1) tamoxifen for 2 years, (2) goserelin (26 monthly subcutaneous injections), (3) tamoxifen plus goserelin, or (4) no endocrine therapy. Some patients electively received tamoxifen or did not and were randomized to only the goserelin option. The study protocol also permitted the use of elective adjuvant chemotherapy. A total of 2,631 women (56% node-negative) were studied. ER status was available in 1,577 (60%).
At a median follow-up of 4.3 years, fewer recurrences were observed among patients who were randomized to receive goserelin than among those randomized to not receive goserelin-261 (20%) vs 330 (24.9%); hazard ratio (HR) = 0.77; 95% confidence interval (CI) = 0.66–0.90; P = .001. This effect was most pronounced among women who were known to have ER-positive disease. The benefit of goserelin appeared to be somewhat less pronounced among those who received concurrent adjuvant tamoxifen or adjuvant chemotherapy, but the differences compared to patients who did not receive concurrent treatments were not statistically significant. The investigators also noted a trend toward fewer deaths among women allocated to receive goserelin-140 (10.7%) vs 165 (12.4%); HR = 0.84; 95% CI = 0.67–1.05; P =.12.
Thus, in this study, medical castration with goserelin for 2 years in premenopausal patients with ER-positive disease produced a statistically significant benefit in terms of DFS, and a trend toward improvement in OS, irrespective of concurrent adjuvant tamoxifen or chemotherapy.[10]
E5188 Trial
The study from the ECOG,[11] conducted by Davidson and colleagues, also examined the role of goserelin with or without tamoxifen in premenopausal women. In this study, 1,503 eligible premenopausal node positive women who all received CAF chemotherapy were randomized to either goserelin alone (CAFZ) or goserelin plus tamoxifen (CAFZT) for 5 years. The use of CAFZT in comparison to CAFZ was associated with significantly better DFS (P < .01) and time to recurrence (TTR) (P < .01) but not a significant difference in OS (P =.23). The addition of goserelin alone to CMF (cyclophosphamide, methotrexate, 5-FU) showed only a trend toward improved DFS (P =.15), TTR (P =.17), and OS (P =.14).
A preliminary hypothesis-generating analysis presented by Dr. Davidson, however, suggested that the addition of goserelin had a greater effect in younger women and/or in women who did not become postmenopausal as a result of chemotherapy, whereas the addition of tamoxifen seemed more effective in older women and/or in women who became menopausal as a result of their chemotherapy. This hypothesis remains to be further explored and substantiated in other prospective randomized trials.

A more complete and updated meta-analysis published in The Lancet in May 2007 obtained data from 11,906 premenopausal women with early breast cancer randomized in 16 trials.[12] In this meta-analysis, LHRH agonists, when used as the only systemic adjuvant treatment, did not significantly reduce recurrence (28.4% relative reduction [RR]; 95% CI consistent with a 50.5% reduction to a 3.5% increase; P =.08) or death after recurrence (17.8% RR; 95% CI = 52.8% reduction to 42.9% increase, P = .49) in hormone receptor–positive cancers. The addition of LHRH agonists to tamoxifen, chemotherapy, or both reduced recurrence by 12.7% (95% CI = 2.4% RR to 21.9% RR, P =.02), and death after recurrence by 15.1% (95% CI = 1.8% RR to 26.7% RR, P =.03). LHRH agonists were ineffective in hormone receptor–negative tumors.
In this 2007 meta-analysis the results of the EBCTCG’s previous meta-analysis were both supported and refined. The number of patients included in trials of LHRH agonists was substantially larger than that available in the previous published Overview.[13] The results broadly supported those of previous analyses but also showed new and important details. It is of particular interest that in this 2007 meta-analysis, the LHRH agonists benefit women younger than 40 after chemotherapy but not older premenopausal women.
Chemotherapy Compared to Ovarian Ablation With or Without Tamoxifen
A number of trials have directly compared chemotherapy of various types to ovarian ablation/suppression with or without the addition of tamoxifen in premenopausal women.
Scottish investigators evaluated premenopausal node-positive and node-negative women randomized to receive three weekly intravenous CMF chemotherapy for eight cycles compared to ovarian removal. The study revealed no significant difference in the effects of CMF vs ovarian removal on either DFS or OS. Patients with ER levels greater than 100 fmoles/mg, however, had a better DFS and OS with ovarian removal. In contrast, those with ER levels under 100 fmoles/mg did better with CMF.[14] While this CMF chemotherapy schedule may not be optimal,[15] the study suggests that ovarian removal for women with high levels of ER may be at least as effective as some types of CMF chemotherapy.
Ejlertson and colleagues[16] compared ovarian ablation by irradiation to intravenous CMF for nine cycles in 732 premenopausal women who had ER-positive tumors, were node-positive, and/or had tumors larger than 5 cm. The 5-year DFS was superior for ovarian ablation, but OS was equivalent in the two treatment groups.
Two important large trials involving an LHRH analog were published in the Journal of Clinical Oncology (JCO) in 2002.[17,18] In the first of these studies, conducted by Jakesz and colleagues,[17] 1,095 women with stage I/II, ER- and/or PR-positive breast cancer were randomized to receive goserelin plus tamoxifen compared to CMF chemotherapy. Women receiving the endocrine therapy had a significantly improved DFS (P < .02), but OS was not significantly different.
The other landmark study published in the same issue of JCO was the Zoladex Early Breast Cancer Research Association (ZEBRA) trial conducted by Jonat et al.[18] In this study, 1,640 pre- and perimenopausal node-positive women whose tumors were ER-positive or -negative were randomized to receive goserelin for 2 years vs CMF for six cycles. In ER-positive women, DFS and OS were equivalent, whereas in ER-negative women, DFS and OS were superior in the CMF arm. In both the Jakesz and Jonat trials, women who became amenorrheic following CMF therapy had better outcomes than those who did not, suggesting that the endocrine effects of chemotherapy also play a role in this setting.

Several other randomized trials have compared CMF to ovarian suppression with or without tamoxifen,[19-21] and two have investigated FAC or FEC vs ovarian suppression plus tamoxifen,[22] all with similar results suggesting equivalence or superiority for the endocrine arm (see Table 1).
Nevertheless, with the development of second- and third-generation combinations such as CEF,[23] dose-dense AC/paclitaxel,[24] and dose-dense EC/paclitaxel[25]-which are more efficacious than standard Bonadonna CMF or AC alone-it is unclear what conclusions one should draw from studies that compare “first-generation” chemotherapies such as AC or CMF to ovarian ablation by any means.[26] In the 2007 meta-analysis published in The Lancet,[12] LHRH agonists showed similar efficacy to chemotherapy in terms of recurrence (3.9% increase; 95% CI = 7.7% RR to 17.0% increase) and death (6.7% RR, 95% CI = 20.7% RR to 9.6% increase), but neither difference was significant. No trials had assessed an LHRH agonist vs chemotherapy with tamoxifen in both arms. Furthermore, the Lancet 2007 meta-analysis clearly displays the equivalence of LHRH agonists to chemotherapy in hormone receptor–positive cancers, an equivalence that does not exist in those that are hormone receptor–negative.
TABLE 1
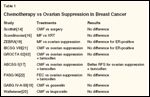
Chemotherapy vs Ovarian Suppression in Breast Cancer
Additional trials and follow-up of previously conducted trials will be required to further clarify this situation. It is also important to examine women receiving chemotherapy who have become amenorrheic, in comparison to those who have not. It may be that the addition of ovarian ablation is important only in women who do not achieve amenorrhea following chemotherapy. Prospective randomized trials of this specific question are currently underway.
In particular, the Suppression of Ovarian Function Trial (SOFT) will ask the question of whether ovarian ablation adds additional benefit in women who have received current standard chemotherapy but have not become amenorrheic. This study led by the International Breast Cancer Study Group (IBCSG) opened in 2004 and is currently accruing patients across Europe, North America, and Australia. The IBCSG has also designed another international trial-the Tamoxifen/Exemestane Trial (TEXT)-in which premenopausal women who undergo ovarian ablation will be randomized to receive either tamoxifen or exemestane (Aromasin), testing the question of whether tamoxifen or an aromatase inhibitor is superior once premenopausal women are made postmenopausal.
In the Premenopausal Endocrine-Responsive Chemotherapy (PERCHE) trial, women who received ovarian ablation and tamoxifen or ovarian ablation and exemestane were being randomized to receive chemotherapy or not, testing the concept of whether chemotherapy adds to optional adjuvant endocrine therapy. Unfortunately, this trial was closed because of poor accrual. Another trial that will clarify this issue is the Trial Assigning IndividuaLized Options for Treatment (Rx), or TAILORx, which will explore the role of the Oncotype DX assay in predicting risk of recurrence and response to chemotherapy in women with ER- and/or PR-positive, node-negative breast cancer.
Quality of Life and Ovarian Ablation/Suppression
There is a close relationship among quality of life, chemotherapy-induced menopause, menopausal symptoms from induced menopause and/or endocrine therapy, and sexuality in breast cancer survivors.[27-30] Young women report worse emotional well-being, more psychological distress, higher anxiety, more unmet needs, and greater concerns about finances, work and self-image than older women.[31-38] Chemotherapy with induced menopause is associated with lower levels of physical functioning and poorer quality of life.[27,36,37,39-41] In addition, psychological distress is associated with the loss of both menstrual function and flexibility in childbearing potential.[28,29,36]. The abrupt menopause brought on by chemotherapy seems to result in more severe vasomotor symptoms[42,43] as well as changes in emotional, physical, and functional well-being.[27,44] Women who did not feel adequately prepared for chemotherapy-induced menopause reported greater uncertainty and psychological distress.[45]
Young women (generally defined as ≤ 40 years old) who receive adjuvant chemotherapy, particularly when they experience drug-induced menopause, are at a greater risk for negative changes in sexuality and poorer sexual-functioning outcomes.[27,29,46-48] Breast cancer survivors who have received systemic adjuvant therapy report less sexual satisfaction,[49] lower levels of sexual function,[47-50] decreased libido,[51] more difficulty reaching orgasm, and more dyspareunia, as well as less sexual satisfaction.[47] These changes in quality of life represent an important area for education, communication, support, and intervention, especially in the very young woman (generally defined as ≤ 35 years old).
In summary, premenopausal women experience exacerbated quality-of-life effects, particularly those related to premature menopausal issues, compared to those over age 50.
Ovarian Ablation/Suppression and the Very Young Woman

While many databases suggest that young age is a poor prognostic factor independent of any other, this is complicated by several additional issues. First, it is well known that women under 35 are much less likely to become amenorrheic following various types of chemotherapy than those who are older.[52,53] Since it is known that hormone-sensitive premenopausal patients may gain much of the positive effect of chemotherapy from endocrine ablation, this younger group may be adversely affected by the fact that they do not develop ovarian suppression following chemotherapy.[54]
This point of view has been supported by the work of the IBCSG, in which younger patients with ER-positive tumors had a significantly worse prognosis than younger patients with ER-negative disease (25% vs 47% 10-year disease-free survival; HR = 1.49, P = .014), particularly those who did not achieve amenorrhea compared with those who experienced some cessation of menses (23% ± 6% vs 38% ± 3%; HR = 1.67; 95% CI = 1.19–2.34; P = .003) in various trials of the timing and duration of adjuvant systemic therapy containing CMF.[52] This observation suggests that the endocrine effects of chemotherapy alone were insufficient therapy for very young women with endocrine-responsive tumors.
In very young women whose tumors are endocrine-responsive, endocrine treatments such as ovarian ablation and tamoxifen may be extremely effective, but the side effects of such extremely premature menopause and/or the use of tamoxifen may also be particularly troublesome in this group of women.
Ovarian Function and Chemotherapy Toxicity
REFERENCE GUIDE
Therapeutic Agents
Mentioned in This Article
Cyclophosphamide
Doxorubicin
Exemestane (Aromasin)
Fluorouracil (5-FU)
Goserelin (Zoladex)
Methotrexate
Tamoxifen
Brand names are listed in parentheses only if a drug is not available generically and is marketed as no more than two trademarked or registered products. More familiar alternative generic designations may also be included parenthetically.
Ovarian toxicity is a predictable side effect of alkylating agent–based chemotherapy and is influenced by the cumulative dose and duration of therapy.[55] Younger women who preserve their menses or who develop reversible amenorrhea will still experience premature menopause as a delayed effect.[56] In the average woman, the perimenopausal transition begins at ≤ 35 years of age, with a gradual decline in mature functional follicles and a decrease in sensitivity to gonadotropin stimulation over the following 10 to 15 years. These changes lead to alterations in bleeding patterns and cycle length, as well as wide variations in hormonal levels. The risk for ovarian failure following chemotherapy is substantially higher in women who are closer to the average age of natural menopause, which is 51 years, because of diminished follicular reserve. Women with chemotherapy-induced ovarian damage will experience menstrual changes, menopausal symptoms, changes in fertility potential, and infertility.
Some good summaries of the incidence and variables of age, dose, and drugs associated with ovarian toxicity in adjuvant chemotherapy for breast cancer have been written.[57] Age is a strong discriminating factor for ovarian failure.[58] The time required to develop amenorrhea also corresponds with age. Higher cumulative doses and longer durations of therapy are associated with the greater risk for amenorrhea. In particularly young women, menses can return following a year or more of amenorrhea.[58,59]
Ovarian Ablation vs Tamoxifen as Adjuvant Therapy
Tamoxifen used alone is also clearly effective as adjuvant therapy for node-negative and lower-risk ER- and PR-positive women, but virtually no data have directly compared its efficacy to chemotherapy or ovarian ablation in the adjuvant setting.
Addition of Tamoxifen or Ovarian Ablation to Chemotherapy
Considerable data now suggest that the addition of tamoxifen to adjuvant chemotherapy adds benefit in premenopausal receptor-positive women,[60-62] and more data will certainly be available in the final 2005 EBCTCG Overview. It is less clear whether ovarian ablation suppression adds significantly to the role of chemotherapy in premenopausal women. The additional benefit of ovarian ablation may be restricted to women who do not become postmenopausal from adjuvant chemotherapy, which may include, in particular, the very young women. The ongoing SOFT study specifically addresses this question.
Financial Disclosure:Dr. Pritchard has been a consultant with Sanofi-Aventis, AstraZeneca, Roche, Pfizer, Ortho-Biotech, YM Biosciences, Biomira, and Novartis. She has received research funding either directly or indirectly through the National Cancer Institute of Canada Clinical Trials Group, contracted with pharmaceutical companies, from AstraZeneca, YM Biosciences, Bristol-Myers Squibb, Sanofi-Aventis, Amgen, Ortho Biotech, Pfizer, and Novartis. She has received honoraria from Sanofi-Aventis, AstraZeneca, Pfizer, Roche, Aegera, YM Biosciences, and Novartis, and has given paid expert testimony for Sanofi-Aventis and Astra-Zeneca. She has been a member of advisory committees for Sanofi-Aventis, AstraZeneca, Ortho Biotech, Roche, Pfizer, Novartis, Aegera, and YM Biosciences.
References:
1. Beatson GT: On the treatment of inoperable cases of carcinoma of the mamma: Suggestions for a new method of treatment with illustrative cases. Lancet 2:104-107, 1896.
2. Jensen EV, Jacobson HI: Basic guides to the mechanism of estrogen action. Recent Prog Horm Rres 18:387-414, 1962.
3. Canadian Cancer Society: Canadian Cancer Statistics, 2008. Available at www.cancer.ca. Accessed December 1, 2008.
4. Early Breast Cancer Trialists’ Collaborative Group: Effects of adjuvant tamoxifen and of cytotoxic therapy on mortality in early breast cancer: An overview of 61 randomized trials among 28,896 women. N Engl J Med 319:1681-1692, 1988.
5. Early Breast Cancer Trialists’ Collaborative Group: Systemic treatment of early breast cancer by hormonal, cytotoxic or immune therapy: 133 randomized trials involving 31,000 recurrences and 24,000 deaths among 75,000 women. Lancet 339:1-15, 71-85, 1992.
6. Pritchard KI: Ovarian ablation as adjuvant therapy for premenopausal women with early breast cancer: Phoenix arisen? (editorial). Lancet 339:95-96, 1992.
7. Taylor CW, Green S, Dalton WS, et al: Multicentere randomized clinical trial of goserelin versus surgical ovariectomy in premenopausal patients with receptor-positive metastatic breast cancer: An Intergroup study. J Clin Oncol 16:994-999, 1998.
8. Klijn JR, Blamey F, Boccardo T, et al: Combined tamoxifen and luteinizing hormone-releasing hormone (LHRH) agonist versus LHRH agonist alone in premenopausal advanced breast cancer: A meta-analysis of four randomized trials. J Clin Oncol 19:343-353, 2001.
9. Arriagada R, Le MG, Spielmann M, et al: Randomized trial of adjuvant ovarian suppression in 926 premenopausal patients with early breast cancer treated with adjuvant chemotherapy. Ann Oncol 16:389-396, 2005.
10. Baum M, Hackshaw A, Houghton J, et al: Adjuvant goserelin in premenopausal patients with early breast cancer: Results from the ZIPP study. Eur J Cancer 42:895-904, 2006.
11. Davidson NE, O’Neill AM, Vukov AM, et al: Chemoendocrine therapy for premenopausal women with axillary lymph node-positive, steroid hormone receptor-positive breast cancer: Results from INT 0101 (E5188). J Clin Oncol 23:5973-5982, 2005.
12. LHRH-agonists in Early Breast Cancer Overview Group: Use of luteinizing-hormone-releasing hormone agonists as adjuvant treatment in premenopausal patients with hormone-receptor-positive breast cancer: A meta-analysis of individual patient data from randomized adjuvant trials. Lancet 369:1711-1723, 2007.
13. Early Breast Cancer Trialists’ Collaborative Group: Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: An Overview of the randomized trials. Lancet 365:1687-1717, 2005.
14. Adjuvant ovarian ablation versus CMF chemotherapy in premenopausal women with pathological stage II breast carcinoma: The Scottish trial. Scottish Cancer Trials Breast Group and ICRF Breast Unit, Guy’s Hospital, London. Lancet 341:1293-1298, 1993.
15. Engelsman E, Klijn JCM, Rubens RD, et al: Classical CMF versus a 3-weekly intravenous CMF schedule in postmenopausal patients with advanced breast cancer. Eur J Cancer 27:966-970, 1991.
16. Ejlertsen B, Dombernowski P, Mouridsen HT, et al: Comparable effect of ovarian ablation (OA) and CMF chemotherapy in premenopausal hormone receptor positive breast cancer patients (PRP) (abstract 248). Proc Am Soc Clin Oncol 18:66a, 1999.
17. Jakesz R, Hausmaninger H, Kubista E, et al: Randomized adjuvant trial of tamoxifen and goserelin versus cyclophosphamide, methotrexate, and fluorouracil: Evidence for the superiority of treatment with endocrine blockade in premenopausal patients with hormone-responsive breast cancer-Austrian Breast and Colorectal Cancer Study Group trial J Clin Oncol 20:4621-4627, 2002.
18. Jonat W, Kaufmann M, Sauerbrei W, et al: Goserelin versus cyclophosphamide, methotrexate, and fluorouracil as adjuvant therapy in premenopausal patients with node-positive breast cancer: The Zoladex Early Breast Cancer Research Association study. J Clin Oncol 20:4628-4635, 2002.
19. von Minckwitz G, Graf E, Geberth M, et al: Goserelin versus CMF as adjuvant therapy for node-negative, hormone receptor-positive breast cancer in premenopausal patients (abstract 534). The GABG IV-A-93 trial. Proc Am Soc Clin Oncol 23:10, 2004.
20. Wallwiener D, Possinger K, Schmid P, et al: A phase III trial comparing adjuvant treatment with leuprorelin acetate 3M-depot for 24 months with CMF chemotherapy in ER/PR + node + pre-perimenopausal breast cancer patients (abstract 533). Proc Am Soc Clin Oncol 23:10, 2004.
21. Bernhard J, Zahrieh D, Castiglione-Gertsch M, et al: Adjuvant chemotherapy followed by goserelin compared with either modality alone: The impact on amenorrhea, hot flashes, and quality of life in premenopausal patients. The International Breast Cancer Study Group Trial VIII. J Clin Oncol 25:263-270, 2007.
22. Roche H, Kerbrat P, Bonneterre J, et al: Complete hormonal, blockade versus chemotherapy in premenopausal early-stage breast cancer patients (pts) with positive hormone receptor (HR+) and 1-3 node-positive (N+) tumor: Results of the FASG 06 trial (abstract 279). Proc Am Soc Clin Oncol 19:72a, 2000.
23. Levine MN, Bramwell VH, Pritchard KI, et al: A randomized trial of cyclophosphamide, epirubicin, fluorouracil chemotherapy compared with cyclophosphamide, methrotrexate, fluorouracil in premenopausal women with node positive breast cancer. J Clin Oncol 16:2651-2658, 1998.
24. Citron M, Berry DA, Cirrincione C, et al: Randomized trial of dose-dense versusu conventionally scheduled and sequential versus concurrent combination chemotherapy as postoperative adjuvant treatment of node-positive primary breast cancer: First report of Intergroup trial C9741/Cancer and Leukemia Group B trial 9741. J Clin Oncol 21:1431-1439, 2003.
25. Burnell M, Levine M, Chapman JA, et al: A randomized trial of CEF versus dose dense EC followed by paclitaxel versus AC followed by paclitaxel in women with node positive or high risk node negative breast cancer, NCIC CTG MA.21: Results of an interim analysis (abstract A-53). Breast Cancer Res Treat 100(suppl 1), 2006.
26. Pritchard KI: Adjuvant therapy for premenopausal women with breast cancer: Is it time for another paradigm shift? J Clin Oncol 20:4611-4614, 2002.
27. Ganz PA, Rowland JH, Desmond K, et al: Life after breast cancer: Understanding women’s health-related quality of life and sexual functioning. J Clin Oncol 16:501-514, 1998.
28. Knobf MT: Carrying on: The experience of premature menopause in women with early stage breast cancer. Nurs Res 51:9-17, 2002. 29. Wilmoth M: The aftermath of breast cancer: An altered sexual self. Cancer Nurs 24:278-286, 2001.
30. Dow K, Ferrell BR, Haberman M: The meaning of quaIity of life in cancer survivorship. Oncol Nurs Forum 26:519-528, 1999. 31. Ganz PA, Greendale GA, Petersen L, et al: Breast cancer in younger women: Reproductive and late health effects of treatment. J Clin Oncol 21:4184-4193, 2003.
32. Goodwin PJ, Ennis M, Pritchard K, et al: Association of young age and chemotherapy with psychosocial distress and health-related quality of life (HRQOL) during the first year after breast cancer (BC) (abstract 8014). Proc Am Soc Clin Oncol 22:728, 2004.
33. Bloom JR, Kessler L: Risk and timing of counseling and support intervention for younger women with breast cancer. JNCI Monogr 16:199-206, 1994.
34. Vinokur A, Threatt B, Vinokur-Kaplan D: The process of recovery from breast cancer for younger and older patients: Changes during the first year. Cancer 65:1242-1254, 1990.
35. Mor V, Malin M, Allen S: Age differences in the psychosocial problems encountered by breast cancer patients. J Natl Cancer Inst Monogr 16:191-197, 1994.
36. Spencer S, Lehman J, Wynings C: Concerns about breast cancer and relations to psychosocial well-being in a multiethnic sample of early-stage patients. Health Psychology 18:159-168, 1999.
37. Arora N, Gustafson D, Hawkins R: Impact of surgery and chemotherapy on teh quality of life of younger women with breast carcinoma: A prospective study. Cancer 92:1288-1298, 2001.
38. Kroenke CH, Rosner B, Chen WY, et al: Functional impact of breast cancer by age at diagnosis. J Clin Oncol 22:1849-1856, 2004.
39. Ganz PA, Kwan L, Stanton AL, et al: Quality of life at the end of primary treatment of breast cancer: First results from the Moving Beyond Cancer randomized trial. J Natl Cancer Inst 96:376-387, 2004.
40. Hürny C, Bernhard J, Coates AS, et al: Impact of adjuvant therapy on quality of life in women with node-positive operable breast cancer. International Breast Cancer Study Group. Lancet 347:1279-1284, 1996.
41. Schag CA, Ganz PA, Polinsky ML, et al: Characteristics of women at risk for psychosocial distress in the year after breast cancer. J Clin Oncol 11:783-793, 1993.
42. Carpenter J, Johnson DH, Wagner L, et al: Hot flashes and related outcomes in breast cancer survivors and matched comparison women. Oncol Nurs Forum 29:E16-E25, 2002.
43. Carpenter J, Elam JL, Ridner SH, et al: Sleep, fatigue and depressive symptoms in breast cancer survivors and matched healthy women experiencing hot flashes. Oncol Nurs Forum 31:591-598, 2004.
44. Couzi RJ, Helzlsouer KJ, Fetting JH: Prevalence of menopausal symptoms among women with a history of breast cancer and attitudes toward estrogen replacement therapy. J Clin Oncol 13:2737-2744, 1995.
45. Knobf MT: The influence of symptom distress and preparation on responses of women with early stage breast cancer to induced menopause. Psycho-Oncology 8:88a, 1999.
46. Ganz PA, Coscarelli A, Fred C: Breast cancer survivors: Psychosocial concerns and quality of life. Breast Cancer Res Treat 38:183-199, 1996.
47. Ganz PA, Desmond KA, Belin TR, et al: Predictors of sexual health in women after a breast cancer diagnosis. J Clin Oncol 17:2371-2380, 1999.
48. Lindley C, Vasa S, Sawyer WT, et al: Quality of life and preferences for treatment following systemic adjuvant therapy for early-stage breast cancer. J Clin Oncol 16:1380-1387, 1998.
49. Dorval M, Maunsell E, Deschenes L, et al: Long-term quality of life after breast cancer: Comparison of 8-year survivors with population controls. J Clin Oncol 16: 487-494, 1998.
50. Ganz PA, Desmond KA, Leedham B, et al: Quality of life in long-term disease-free survivors of breast cancer: A follow-up study. J Natl Cancer Inst 94:39-49, 2002.
51. Barton D, Wilwerding M, Carpenter L, et al: Libido as part of sexuality in female cancer survivors. Oncol Nurs Forum 31:599-607, 2004.
52. Pagani O, O’Neil A, Castiglione M, et al: Prognostic impact of amenorrhea after adjuvant chemotherapy in premenopausal breast cancer patients with axillary node involvement: Results of the International Breast Cancer Study Group (IBCSG) Trial VI. Eur J Cancer 34:632-640, 1998.
53. Parulekar W, Day AG, Ottaway JA, et al: Incidence and prognostic impact of amenorrhea during adjuvant therapy in high-risk premenopausal breast cancer: Analysis of a National Cancer Institute of Canada Clinical Trials Group study-NCIC CTG MA.5. J Clin Oncol 23:6002-6008, 2005.
54. Aebi S, Gelber R, Castiglione-Gertsch M: Is chemotherapy alone adequate for young women with estrogen-receptor-positive breast cancer. Lancet 365:1869-1874, 2000.
55. Averette HE, Boike GM, Jarrell MA: Effects of cancer chemotherapy on gonadal function and reproductive capacity. Cancer J Clin 40:199-209, 1990.
56. Meirow D: Reproduction post-chemotherapy in young cancer patients. Mol Cell Endocrinol 169:123-131, 2000.
57. Knobf MT: The influence of endocrine effects of adjuvant therapy on quality of life outcomes in younger breast cancer survivors. Oncologist 11:96-110, 2006.
58. Goodwin PJ, Ennis M, Pritchard KI, et al: Risk of menopause during the first year after breast cancer diagnosis J Clin Oncol 17:2365-2370, 1999.
59. Bonadonna G, Valagussa P, Rossi A, et al: Ten-year experience with CMF-based adjuvant chemotherapy in resectable breast cancer. Breast Cancer Res Treat 5: 95-115, 1985.
60. Andersson M, Kamby C, Jensen MB, et al: Tamoxifen in high-risk premenopausal women with primary breast cancer receiving adjuvant chemotherapy. Report from the Danish Breast Cancer Co-operative Group DBCG 82B Trial. Eur J Cancer 35:1659-1666, 1999.
61. Castiglione-Gertsch M, Price K, Nasi ML, et al: Is the addition of adjuvant chemotherapy always necessary in node negative postmenopausal breast cancer patients who receive tamoxifen? First results of the IBCSG Trial IX (abstract 281). Proc Am Soc Clin Oncol 19:73A, 2000.
62. Castiglione-Gertsch M, O’Neill A, Gelber RD, et al: Is the addition of adjuvant chemotherapy always necessary in node negative pre/perimenopausal breast cancer patients who receive goserelin? First results of IBCSG trial VIII (abstract 149). Proc Am Soc Clin Oncol 21:38a, 2002.
63. Boccardo F, Rubagotti A, Amoroso D, et al: Cyclophosphamide, methotrexate, and fluorouracil versus tamoxifen plus ovarian suppression as adjuvant treatment of estrogen receptor-positive pre-/perimenopausal breast cancer patients: Results of the Italian Breast Cancer Adjuvant Study Group 02 randomized trial. J Clin Oncol 18:2718-2727, 2000.