An Overview of Chemotherapy-Related Cognitive Dysfunction, or ‘Chemobrain’
This review will focus largely on the effects of systemic cytotoxic treatment on cognitive function, reflecting what has been most extensively studied in the literature.
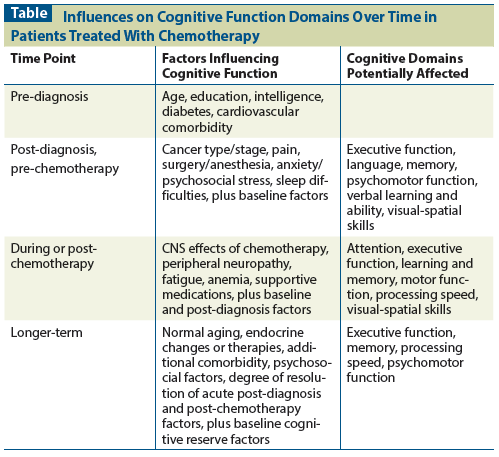
Table: Influences on Cognitive Function Domains Over Time in Patients Treated With Chemotherapy
Chemotherapy can improve prospects for long-term survival after a cancer diagnosis, but it may also be associated with long-term toxicity, including the possibility of cognitive dysfunction. While a variety of factors may contribute to cognitive impairment in cancer survivors, there is increasing evidence that chemotherapy contributes to both subjective and objective changes in cognition. These effects appear to be most pronounced in the short-term, with improvement over time expected for most patients. Pharmacologic treatments do not have proven value in the management of chemotherapy-related cognitive dysfunction, but patients may benefit from cognitive rehabilitation. Clinical guidelines are needed for assessment and management of chemotherapy-related cognitive dysfunction.
Introduction
Chemotherapy-associated cognitive dysfunction, often referred to as “chemobrain,” includes subjectively reported and objectively measured problems with cognition following chemotherapy. The American Cancer Society estimates there will be close to 14.5 million cancer survivors in the United States by 2015.[1] Increasingly, individuals with cancer are experiencing long-term survival following initial treatment, leading to an ever greater need to understand, manage, and prevent lasting adverse effects of cancer treatment. Reflecting this need, the American Society of Clinical Oncology has recently issued a set of new Survivorship Guidelines addressing fatigue, mood disorders, and peripheral neuropathy. Currently there are no clear guidelines for the clinical assessment and management of chemobrain.
The recognition that a variety of factors other than chemotherapy may contribute to cognitive decline in cancer patients has led to use of the broader term, “cancer treatment–related cognitive impairment.” This review, however, will focus largely on the effects of systemic cytotoxic treatment on cognitive function, reflecting what has been most extensively studied in the literature. It should also be noted that much of the research on this topic has been conducted in breast cancer patients, as they represent the largest group of long-term cancer survivors who have had frequent exposure to chemotherapy. This review does not address cognitive effects of central nervous system (CNS) malignancies or CNS-directed treatments.
In a sampling of participants in the National Health and Nutrition Examination Survey, individuals with and without a history of cancer were surveyed about whether they felt they were limited by difficulty with memory or periods of confusion. Approximately 14% of cancer survivors reported memory problems, compared with 8% of participants without a prior cancer diagnosis, representing an approximately 40% increase in the likelihood of cancer survivors reporting cognitive concerns.[2] Similarly, studies assessing cognitive function with objective cognitive tests have suggested a higher likelihood of impairment among individuals who have received chemotherapy. In one study of breast cancer survivors who had, on average, completed treatment with adjuvant cyclophosphamide, methotrexane, and fluorouracil (CMF) chemotherapy more than 20 years prior to enrollment, cognitive deficits were observed compared with a control group who underwent the same neuropsychological tests as part of a population-based assessment.[3]
Factors Other Than Chemotherapy That Have an Impact on Cognitive Function
Studies that only address cognitive performance in a cross-sectional manner after patients have completed chemotherapy do not account for other effects of the cancer diagnosis and treatment or possible baseline differences between patients who get cancer and those who do not. To get around these limitations, investigators have begun to conduct studies that perform baseline cognitive assessments and include, in addition to healthy control groups, control groups of patients with cancer types similar to those under investigation but treated without systemic therapy. In some studies, formal testing of cancer patients after initial diagnosis and surgery but prior to any systemic therapy has demonstrated higher-than-expected baseline rates of cognitive dysfunction. Among breast cancer patients who had completed surgery but had not yet initiated planned chemotherapy, cognitive dysfunction rates upwards of 20% have been reported in several studies.[4-6] In a study of colon cancer patients evaluated prior to adjuvant chemotherapy, 37% demonstrated cognitive impairment prior to systemic treatment,[7] and among testicular cancer patients evaluated after surgery but prior to chemotherapy, the incidence of cognitive impairment was 46%.[8] Among studies looking at specific cognitive domains affected in newly diagnosed cancer patients, abnormalities have been seen in psychomotor function, verbal learning and ability, executive function, language, visual-spatial skill, and memory.[4-6,8,9]
There is evidence that surgery and anesthesia contribute to cognitive impairment, particularly in older patients, those with cardiovascular disease, and patients who undergo more extensive surgical procedures or have post-surgery complications. In general, postoperative cognitive dysfunction, believed to be due in part to inflammatory and immune responses to surgery, appears to resolve within days to months.[10]
It is unclear to what extent baseline cognitive impairment is related to anxiety and depression. Certainly learning of a cancer diagnosis can affect mood, which may in turn affect sleep and other physiologic functions. In a small study of breast cancer patients who were to receive either chemotherapy or radiation treatment, pretreatment worry was associated with alterations in brain function measured by functional magnetic resonance imaging (MRI), and with subjective and objective measures of cognitive function in both treatment groups, with the prechemotherapy group reporting significantly more worry.[11] However, in several studies of women undergoing adjuvant or neoadjuvant chemotherapy for breast cancer, the authors reported that anxiety, distress, or poor quality of life correlated with self-perceived cognitive concerns but not with neuropsychological test results.[6,9,12] In another study of breast cancer patients, cognitive impairment observed prior to systemic treatment was associated with certain comorbidities but not with anxiety, depression, or type of surgery.[13]
In addition to any modest effects on baseline test performance, mood may influence cognitive function assessment during or after chemotherapy treatment. However, these effects seem to be predominantly in subjective rather than objective performance. In a multicenter study of 101 breast cancer patients undergoing chemotherapy, negative affectivity and depression correlated with self-reported cognitive dysfunction.[14] In another study of 53 breast cancer survivors at least 2 years out from diagnosis, subjective cognitive complaints were associated with measures of fatigue and psychological distress but not with objective performance on cognitive testing.[15] Similarly, as discussed above, in a study of 40 women receiving adjuvant or neoadjuvant chemotherapy for breast cancer, the authors found anxiety, depression, and poor quality of life correlated with self-perceived cognitive concerns but not with neuropsychological test results.[12]
Endocrine factors may influence objective neuropsychological testing performance after chemotherapy; however, results have been mixed. In a longitudinal study of breast cancer patients undergoing chemotherapy for early-stage breast cancer, treatment-induced menopause was associated with some decline in cognitive performance following chemotherapy.[16] In another longitudinal study of cognitive effects of chemotherapy for breast cancer, use of adjuvant endocrine therapy was associated with worse performance on measures of processing speed and verbal memory.[17] In a prospective study of women receiving adjuvant chemotherapy for breast cancer, fatigue and menopausal symptoms correlated with quality-of-life scores but not with cognitive impairment as determined by a High-Sensitivity Cognitive Screen.[18]
Assessment of Cognitive Function
Correlations between subjective reports of cognitive impairment and results of neuropsychological testing have been inconsistent, making it challenging to assess for the presence of cognitive dysfunction. Several studies have found that patients reporting subjective cognitive impairment during or following chemotherapy do not show a decline in neuropsychological testing performance.[15,16,19,20] Other studies that did demonstrate apparent effects of chemotherapy on neuropsychological cognitive testing also found no correlation between those results and patients’ subjective concerns.[3,12,21] Additional studies have demonstrated selective relationships between patient-reported cognitive concerns and neuropsychological test results. For example, three studies in breast cancer patients showed self-perceived cognitive dysfunction was correlated with impairment of memory but not with other formally tested cognitive domains.[22-24]
While there is a growing body of literature on neuroimaging and electroencephalogram changes as indicative of “chemobrain,” these tests currently have no role in the clinical diagnosis of chemotherapy-associated cognitive dysfunction. According to a publication by the International Cognition and Cancer Task Force, “objective [neuropsychological] tests remain the gold standard for measuring cognitive function” in the evaluation of cognitive effects of cancer therapies.[25] This task force provided recommendations regarding specific tests that should be included in clinical trials assessing cancer treatment–related cognitive dysfunction. The Hopkins Verbal Learning Test–Revised, the Trail Making Test, and the Controlled Oral Word Association of the Multilingual Aphasia Examination were recommended as reliable and sensitive tests that measure learning and memory, processing speed, and executive function. Because patient reports of cognitive function have not been validated as a reliable way to assess cognitive dysfunction, such subjective measures were believed to be less useful for research purposes.[25]
Patient-reported measures of cognitive impairment are, however, extremely important in clinical practice and are more likely to impact quality of life than deficits uncovered by standardized cognitive testing. Objective neurocognitive assessments may not be as sensitive as patient-perceived changes or may not be testing the specific domains affected in an individual patient. Currently, there are no specific diagnostic criteria for chemotherapy-related cognitive impairment. Similar to assessing fatigue and depression, the patient-reported measures are key in clinical care, and there is currently a need for standardized patient-reported measures of cognitive function for use in daily practice.
Neuropsychological impairment attributable to chemotherapy.
Studies that employ standardized neuropsychological testing and include an untreated control group and/or baseline assessments provide information about the cognitive effects that can be attributed largely to chemotherapy treatment. There is strong evidence that chemotherapy can cause short-term impairment in cognitive function. Longer-term studies have had mixed findings.
Cross-sectional studies that have compared individuals treated with chemotherapy to healthy control populations at various time points have suggested that some chemotherapy regimens lead to impaired cognitive function. Moderate to severe cognitive impairment based on the High-Sensitivity Cognitive Screen was observed in 16% of patients receiving various adjuvant or neoadjuvant chemotherapy regimens for breast cancer, compared with 4% in a healthy control group. In this study, differences between patients and controls appeared to be greater in the cognitive domains of language, attention, concentration, and self-regulation than in the domains of memory and visual-motor performance.[18] In a study of the effects of chemotherapy for non-Hodgkin lymphoma (NHL) on cognitive function, 30 lymphoma patients and 10 healthy controls were assessed within 3 months of completing first-line chemotherapy with a regimen of either rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone (R-CHOP) or bendamustine plus rituximab. Chemotherapy patients receiving bendamustine with rituximab had lower performance scores on objective cognitive testing than controls and R-CHOP–treated patients, whose scores were similar to those of population norms.[26] In a cohort of women between 3 and 10 years post completion of doxorubicin and cyclophosphamide chemotherapy with or without a taxane, breast cancer survivors performed worse on memory testing but otherwise demonstrated a similar performance on neuropsychological testing[23] compared with healthy controls matched for age and education. In a study of long-term breast cancer survivors who had received adjuvant CMF chemotherapy over 20 years earlier on average, impairment in verbal memory, processing speed, executive function, and psychomotor speed was observed compared with healthy controls.[3]
Cross-sectional studies using control populations of cancer patients who did not receive chemotherapy may be a better way to assess any effects of chemotherapy on cognitive function compared with using a healthy control population. One such study compared women who had completed surgery and radiation for breast cancer with those who had completed surgery, radiation, and chemotherapy. Cognitive assessments performed at about 6 months after treatment completion demonstrated no significant differences between the two groups in episodic memory, attention, complex cognition, motor performance, or language.[27] A study that evaluated breast cancer survivors who were at least 2 years out from chemotherapy demonstrated impairment in several cognitive domains compared with survivors who had local therapy without chemotherapy; notably, however, cognitive performance among the chemotherapy recipients was similar to that of a healthy control population.[15] In a relatively long-term follow-up study of breast cancer survivors, neuropsychological assessments were done at various time intervals in women treated with fluorouracil, epirubicin, and cyclophosphamide (FEC) chemotherapy with or without high-dose cyclophosphamide, thiotepa, and carboplatin; CMF chemotherapy; or no chemotherapy. Patients were initially assessed at an average of 2 years after completion of treatment, with repeat assessment approximately 4 years after treatment completion. At the initial 2-year assessment, an increased incidence of cognitive impairment was observed in all chemotherapy-treated groups compared with the control group. The incidence of cognitive impairment was greatest with high-dose chemotherapy, at about 32%, followed by a 20% incidence of cognitive impairment in the CMF group and 17% in the FEC group, compared with 6% in breast cancer patients who did not receive chemotherapy. At the time of the follow-up assessment approximately 2 years later (ie, 4 years post completion of chemotherapy), differences in the rate of cognitive impairment were no longer significant between the groups.[28] In another study, which looked at longer-term effects of chemotherapy in breast cancer and lymphoma survivors who were free of disease at least 5 years out from diagnosis, individuals who received chemotherapy were compared with those who received local therapy without chemotherapy. In that study, cognitive scores were lower in the chemotherapy group, particularly in the cognitive domains of verbal memory and psychomotor functioning.[29]
Studies in which baseline assessments are obtained prior to any chemotherapy treatment allow patients to serve as their own controls for assessing cognitive changes. Several of these studies performed in breast cancer patients[30,16,20] and one in colon cancer patients[31] receiving adjuvant chemotherapy demonstrated no significant change between pre- and post-treatment cognitive assessments. The majority of longitudinal studies, however, have demonstrated pre- to post-treatment decline in at least some cognitive domains acutely following chemotherapy. Specific cognitive domains that have been observed to be adversely affected by chemotherapy in the short-term include executive function,[5,14] motor function,[6,9,32] learning and memory,[5-7,32] processing speed,[5,33] attention,[6,32] and visuospatial skills.[6] In a meta-analysis of 17 cognitive function studies in breast cancer survivors treated with standard-dose chemotherapy, in which baseline testing or a control population was used for comparison, chemotherapy treatment was associated with deficits in verbal and visuospatial abilities, but the magnitude of the effect was small. Differences were not observed in attention, information processing, motor speed, verbal memory, or visual memory.[34]
Relatively few longitudinal studies have formally assessed cognitive function at more than a year following completion of chemotherapy treatment. In a study of 85 women receiving chemotherapy for early-stage breast cancer, control groups were also included and consisted of 49 healthy women and 43 women with newly diagnosed breast cancer who were not receiving chemotherapy. Subjects underwent neuropsychological testing at baseline, at 6 months or at completion of chemotherapy, and at 18 months. No significant differences in cognitive performance between the three groups were observed over time when controlled for age and intelligence.[16] Another longitudinal study of early-stage breast cancer patients that also included a chemotherapy group, a control group of breast cancer patients not receiving chemotherapy, and a healthy control group, assessed processing speed and other cognitive measures at baseline and at 1, 6, and 18 months after treatment. All three groups actually demonstrated improvement in processing speed over time, although the chemotherapy-exposed group did not begin to improve until the second post-chemotherapy time point, suggesting that chemotherapy may have counteracted the learning effect that likely accounted for the improvements in the other two groups. In addition, older patients and patients with low reading scores who received chemotherapy experienced a decline in processing speed scores at the first post-treatment assessment. By 18 months after treatment, however, chemotherapy-treated patients had improvement compared to baseline in all of the cognitive domains assessed.[33] A study that used a High-Sensitivity Cognitive Screen to assess cognitive function in patients undergoing adjuvant chemotherapy for breast cancer compared with a healthy control group found a 16% incidence of moderate to severe cognitive dysfunction in the chemotherapy group compared with 5% in the controls. Assessments 1 and 2 years later demonstrated that these rates had improved, coming down to 4.4% and 3.8%, respectively, in the chemotherapy group and to 3.6% and 0 %, respectively, in the control group. Because the initial testing was done near the end of chemotherapy, it is impossible to know what the baseline differences were between the treatment and control groups.[35] All three of these studies provide reassuring evidence that the acute measurable cognitive impairment associated with chemotherapy diminishes significantly with time.
Correlative Tests Suggesting CNS Effects of Chemotherapy
Correlative studies demonstrating CNS changes associated with chemotherapy provide further evidence of the effects of this treatment modality on the central nervous system. Structural MRI studies have demonstrated a reduction in gray matter density in breast cancer patients exposed to chemotherapy; this appears to improve over time after recovery from chemotherapy.[23,36] White matter changes have also been observed in association with chemotherapy in a study using the MRI technique of diffusion tensor imaging.[32] In addition, functional MRI studies have demonstrated that patients with prior chemotherapy exposure have a reduced activation of certain brain regions during performance of a cognitive task, compared with baseline results[37] or healthy controls.[23] In another study of functional MRI, a subset of patients were more likely to have an increase in brain activation during a working memory task following chemotherapy compared with baseline and those changes correlated with improvement in processing speed scores on neurocognitive testing.[38]
Electroencephalography (EEG) has also been investigated as a correlate of chemotherapy-related cognitive dysfunction. EEG recordings in patients undergoing chemotherapy for NHL did not differ significantly from results in an untreated healthy control population.[26] In a study of 26 breast cancer survivors previously treated with CMF chemotherapy and compared with 23 survivors who did not receive chemotherapy, participants performed information-processing tasks while undergoing concurrent EEG monitoring. Differences in EEG amplitudes of event-related potentials, which relate to intensity of neural structure activation, and latencies, which relate to timing of activation, were observed between survivors who had received chemotherapy and those who had not. While the findings suggest a potential role for EEG as a noninvasive means of evaluating CNS effects of chemotherapy treatment, the changes did not actually correlate with cognitive complaints or formal evaluations of information-processing abilities.[21] In a separate pilot study, patients with breast cancer underwent EEG monitoring and neuropsychological testing before, during, and after recovery from chemotherapy, and age-matched healthy controls were evaluated at the same time points. EEG changes demonstrating increased activity occurred only in patients during chemotherapy and correlated with cognitive complaints but not with objective measures of cognitive function.[20]
Cognitive Compensation
A concept that may explain some of the discrepancies observed between correlative tests, subjective cognitive complaints, and formal neuropsychological testing results is that of cognitive compensation. Individuals with adequate cognitive reserve may have the ability to overcome any detrimental effects of chemotherapy with an increase in activity in some parts of the brain. This increased effort to perform cognitive tasks may be reflected in more perceived cognitive concerns. In the above-described pilot study evaluating EEG as a correlate of cognitive effects of chemotherapy, the alterations in brain activity observed on EEG were heightened following mental and physical tasks in patients undergoing chemotherapy treatment only, perhaps reflecting an increase in effort to maintain the good performance that was observed on objective measures of cognitive function.[20] In a study using functional MRI as a correlate of cognitive function in women experiencing chemotherapy-induced amenorrhea, compared with postmenopausal women receiving chemotherapy as well as premenopausal and postmenopausal controls, all subjects underwent neurocognitive testing and functional MRI during a memory task, to enable the investigators to assess patterns of brain activation. Postmenopausal women receiving chemotherapy exhibited some decline in verbal and visual memory 1 month after chemotherapy treatment, while the women who experienced amenorrhea with chemotherapy demonstrated some improvement in these areas. The latter group also exhibited an increased magnitude of neural activity on the functional MRI studies, suggesting this younger group was able to compensate for the effects of chemotherapy with a change in neural activity. Although the healthy pre- and postmenopausal controls also demonstrated some improvement in cognitive testing scores over time, presumably due to practice effects, heightened neural activity was not observed in this group.[38]
It is likely that patients with more cognitive reserve would have a greater ability to compensate for adverse cognitive effects of chemotherapy. In a study of breast cancer patients evaluated prior to receiving chemotherapy, objective cognitive impairment was associated with diabetes, cardiovascular comorbidity, older age, nonwhite race, and lower education.[13] Supporting the idea that age and reduced cognitive reserve prior to treatment may influence susceptibility to treatment-associated cognitive dysfunction is a longitudinal study of 123 breast cancer patients and 45 healthy controls in which chemotherapy use was not strongly associated with worsening objective measures of cognitive function following treatment, except in older patients and in those with lower baseline cognitive reserve as measured by a Wide Range Achievement Test 3 reading score.[33] Similarly, patient-reported memory problems have been associated with lower levels of education and income; such problems have also been associated with poorer general health.[2]
Interventions for Chemotherapy-Associated Cognitive impairment
If, indeed, reduced cognitive reserve is contributing to poor cognitive performance, it is rational to recommend that patients attempt to modify other factors that may affect cognitive function. Since subjective reports of cognitive dysfunction have been associated with anxiety, depression, fatigue, and sleep disturbance, it is sensible to first address these potential confounding issues in patients who have cognitive concerns after chemotherapy. It is also important to address whether the patient is taking other medications that may affect cognitive function or contribute to delirium. Similarly, aerobic exercise has potential beneficial effects on cognition, as exercise increases blood flow to the brain.[39] Precise mechanisms underlying the effects of chemotherapy on cognitive function are poorly understood and are likely multifactorial. Specific interventions studied for the treatment of chemotherapy-associated cognitive dysfunction have largely consisted of treatments known to be effective for fatigue or for other types of cognitive impairment.
Modafinil, a neural stimulant approved for treatment of narcolepsy, has been evaluated for cancer treatment–associated cognitive dysfunction. In studies evaluating this drug for treatment of cancer therapy–associated fatigue, results of secondary effects on cognitive function have been variable and inconclusive.[40] Methylphenidate, a more broadly acting neural stimulant, has been evaluated for its ability to preserve cognitive function during chemotherapy for breast cancer. In a small randomized study in breast cancer patients, however, no significant benefit on cognitive function, sleep quality, fatigue, or anxiety/depression was observed with d-methylphenidate vs placebo.[41] In another randomized placebo-controlled trial, Ginkgo biloba (EGb 761 extract, 60 mg bid) administered concurrently with chemotherapy was associated with no differences in subjective or objective measures of cognitive function compared with a control group.[42]
Biofeedback and cognitive-behavioral therapy have also been investigated for reducing chemotherapy-associated cognitive dysfunction. In a feasibility study of 23 women with self-reported cognitive impairment 6 months to 5 years following chemotherapy for breast cancer, EEG biofeedback was evaluated for reducing cognitive dysfunction. Patient-reported measures of cognitive function, sleep quality, fatigue, and anxiety/depression were below population norms at baseline and improved to within the normal population range following 20 sessions of neurofeedback training over a period of 10 weeks.[43] In a pilot study of breast cancer survivors with self-reported cognitive concerns, cognitive-behavioral therapy demonstrated promising improvement in self-reported and objectively measured cognitive function, and in patient quality of life.[44] Another randomized study in a similar population suggested improvements in quality of life and verbal memory performance with cognitive-behavioral therapy, but significant improvement in self-reported cognitive complaints was not seen.[45]
Summary
Currently, there is no standard approach to the management or prevention of chemotherapy treatment–related cognitive dysfunction. Research has been limited by small sample size in studies, lack of a standard means of assessing cognitive function, and challenges in identification of the most appropriate control groups. A variety of factors may contribute to the phenomenon described as “chemobrain” and may include the cancer itself, comorbid medical issues, normal aging, fatigue, anxiety, depression, sleep problems, surgery, and endocrine treatments. Depending on the level of a patient’s cognitive reserve, cognitive performance on neuropsychological testing may or may not be preserved in individuals with subjective complaints of cognitive impairment after chemotherapy. Influences on cognitive function at different time points and the cognitive domains that are potentially affected are outlined in the Table.
Cross-sectional studies evaluating the cognitive function of chemotherapy-treated patients at a single time point fail to take into account factors other than chemotherapy that may be contributing to a patient’s cognitive impairment. Studies using pre-chemotherapy assessments demonstrate a fairly high prevalence of baseline cognitive dysfunction and allow for assessment of change over time. The practice effect of repeat testing, as well as considerations such as age, comorbidity, and cognitive reserve may all influence the trajectory of cognitive changes over time, highlighting the importance of including a control population which ideally consists of cancer patients who did not receive chemotherapy as well as an age-matched healthy control population. While neuropsychological testing is valuable in the research setting, its role in evaluating individual patient concerns is less clear, particularly as patient-reported complaints frequently do not correlate with objective test results.
Interactions between patient- reported cognitive concerns and objective neuropsychological test results are complex, but there is strong evidence that chemotherapy may result in both subjective and objective cognitive dysfunction in the short-term. Memory, attention, psychomotor function, processing speed, and executive function appear to be commonly affected.[39,25] There is also evidence that subsets of patients experience long-term cognitive effects after chemotherapy treatment; however, these effects generally appear to be modest in severity, and for the majority of survivors, cognitive impairment will diminish over time. For patients with neurocognitive concerns, while pharmacologic therapies remain unproven in this setting, both cognitive rehabilitation with neurofeedback training and cognitive behavioral training show a potential for benefit. Also, acknowledgement by physicians that cognitive change may, indeed, be a consequence of chemotherapy treatment, and reassurance that improvement over time is anticipated, can be valuable for patients who may be concerned about the effects they are experiencing.
Financial Disclosure:The author has no significant financial interest or other relationship with the manufacturers of any products or providers of any service mentioned in this article.
References:
1. American Cancer Society. Cancer Treatment and Survivorship Facts & Figures 2014-2015. Atlanta: American Cancer Society; 2014. Available from: http://www.cancer.org/acs/groups/content/@research/documents/document/acspc-042801.pdf. Accessed August 11, 2014.
2. Jean-Pierre P, Winters PC, Ahles TA, et al. Prevalence of self-reported memory problems in adult cancer survivors: a national cross-section study. J Oncol Pract. 2011;8:30-4.
3. Koppelmans V, Breteler MMB, Boogerd W, et al. Neuropsychological performance in survivors of breast cancer more than 20 years after adjuvant chemotherapy. J Clin Oncol. 2012;30:1080-6.
4. Ahles TA, Saykin AJ, McDonald BC, et al. Cognitive function in breast cancer patients prior to adjuvant treatment. Breast Cancer Res Treat. 2008;110:143-52.
5. Wefel JS, Saleeba AK, Buzdar A, Meyers CA. Acute and late onset cognitive dysfunction associated with chemotherapy in women with breast cancer. Cancer. 2010;116:3348-56.
6. Jansen CE, Cooper BA, Dodd MJ, Miaskowski CA. A prospective longitudinal study of chemotherapy-induced cognitive changes in breast cancer patients. Support Care Cancer. 2011;19:1647-56.
7. Cruzado JA, Lopez-Santiago S, Martinez-Marin V, et al. Longitudinal study of cognitive dysfunctions induced by adjuvant chemotherapy in colon cancer patients. Support Care Cancer. 2014;22:1815-23.
8. Wefel JS, Vidrine DJ, Veramonti TL, et al. Cognitive impairment in men with testicular cancer prior to adjuvant therapy. Cancer. 2010;117:190-6.
9. Tager FA, McKinley PS, Schnabel FR, et al. The cognitive effects of chemotherapy in post-menopausal breast cancer patients: a controlled longitudinal study. Breast Cancer Res Treat. 2010;123:25-34.
10. Rundshagen I. Postoperative cognitive dysfunction. Dtsch Arztebl Int. 2014;111:119-25.
11. Berman MG, Askren MK, Jung M, et al. Pretreatment worry and neurocognitive responses in women with breast cancer. Health Psychology. 2014;33:222-31.
12. Biglia N, Bounous VE, Malabaila A, et al. Objective and self-reported cognitive dysfunction in breast cancer women treated with chemotherapy: a prospective study. Eur J Cancer Care. 21: 485-492, 2012.
13. Mandelblatt JS, Stern RA, Luta G, et al. Cognitive impairment in older patients with breast cancer before systemic therapy: Is there an interaction between cancer and comorbidity? J Clin Oncol. 2014;32:1909-18.
14. Hermelink K, Kuchenhoff H, Untch M, et al. Two different sides of ‘chemobrain’: determinants and nondeterminants of self-perceived cognitive dysfunction in a prospective, randomized, multicenter study. Psycho-Oncology. 2010;19:1321-8.
15. Castellon SA, Ganz PA, Bower JE, et al. Neurocognitive performance in breast cancer survivors exposed to adjuvant chemotherapy and tamoxifen. J Clin Exp Neuropsychol. 2004;26:955-69.
16. Jenkins V, Shilling V, Deutsch G, et al. A 3-year prospective study of the effects of adjuvant treatments on cognition in women with early stage breast cancer. Brit J Cancer. 2006;94:828-34.
17. Collins B, Mackenzie J, Stewart A, et al. Cognitive effects of chemotherapy in post-menopausal breast cancer patients 1 year after treatment. Psycho-Oncology. 2009;18:134-43.
18. Tchen N, Juffs HG, Downie FP, et al. Cognitive function, fatigue, and menopausal symptoms in women receiving adjuvant chemotherapy for breast cancer. J Clin Oncol. 2003;21:4175-83.
19. Biglia N, Moggio G, Peano E, et al. Effects of surgical and adjuvant therapies for breast cancer on sexuality, cognitive functions, and body weight. J Sex Med. 2010;7:1891-1900.
20. Moore HCF, Parsons MW, Yue GH, et al. Electroencephalogram power changes as a correlate of chemotherapy-associated fatigue and cognitive dysfunction. Support Care Cancer. 2014;22:2127-31.
21. Kreukels BPC, Schagen SB, Ridderinkhof KR, et al. Electrophysiologic correlates of information processing in breast-cancer patients treated with adjuvant chemotherapy. Breast Cancer Res Treat. 2005;94:53-61.
22. Ganz PA, Kwan L, Castellon SA, et al. Cognitive complaints after breast cancer treatments: examining the relationship with neuropsychological test performance. J Natl Cancer Inst. 2013;105:791-801.
23. Conroy SK, McDonald BC, Smith, DJ, et al. Alterations in brain structure and function in breast cancer survivors: effect of post-chemotherapy interval and relation to oxidative DNA damage. Breast Cancer Res Treat. 2013;137:493-501.
24. Downie FP, Mar Fan HG, Houede-Tchen N, et al. Cognitive function, fatigue, and menopausal symptoms in breast cancer patients receiving adjuvant chemotherapy: evaluation with patient interview after formal assessment. Psycho-Oncology. 2006;15:921-30.
25. Wefel JS, Vardy J, Ahles T, Schagen SB. International Cognition and Cancer Task Force recommendations to harmonise studies of cognitive function in patients with cancer. Lancet Oncol. 2011;12:703-8.
26. Zimmer P, Mierau A, Bloch W, et al. Post-chemotherapy cognitive impairment in patients with B-cell non-Hodgkin lymphoma: a first comprehensive approach to determine cognitive impairments after treatment with rituximab, cyclophosphamide, doxorubicin, vincristine and prednisone or rituximab and bendamustine. Leuk Lymphoma. 2014 Jun 5:1-6. [Epub ahead of print]
27. Donovan KA, Small BJ, Andrykowski MA, et al. Cognitive functioning after adjuvant chemotherapy and/or radiotherapy for early-stage breast carcinoma. Cancer. 2005;104:2499-507.
28. Schagen SB, Muller MJ, Boogerd W, et al. Late effects of adjuvant chemotherapy on cognitive function: a follow-up study in breast cancer patients. Ann Oncol. 2002;13:1387-97.
29. Ahles TA, Saykin AJ, Furstenberg CT, et al. Neuropsychologic impact of standard-dose systemic chemotherapy in long-term survivors of breast cancer and lymphoma. J Clin Oncol. 2002;20:485-93.
30. Debess J, Riis JO, Engebjerg MC, Ewertz M. Cognitive function after adjuvant treatment for early breast cancer: a population-based longitudinal study. Breast Cancer Res Treat. 2010;121:91-100.
31. Andreis F, Ferri M, Mazzocchi M, et al. Lack of a chemobrain effect for adjuvant FOLFOX chemotherapy in colon cancer patients. A pilot study. Support Care Cancer. 2013;21:583-90.
32. Deprez S, Amant F, Smeets A, et al. Longitudinal assessment of chemotherapy-induced structural changes in cerebral white matter and its correlation with impaired cognitive functioning. J Clin Oncol. 2012;30:274-81.
33. Ahles TA, Saykin AJ, McDonald BC, et al. Longitudinal assessment of cognitive changes associated with adjuvant treatment for breast cancer: impact of age on cognitive reserve. J Clin Oncol. 2010;28:4434-40.
34. Jim HSL, Phillips KM, Chait S, et al. Meta-analysis of cognitive functioning in breast cancer survivors previously treated with standard-dose chemotherapy. J Clin Oncol. 2012;30:3578-87.
35. Mar Fan HG, Houede-Tchen N, Yi QL, et al. Fatigue, menopausal symptoms, and cognitive function in women after adjuvant chemotherapy for breast cancer: 1- and 2-year follow-up of a prospective controlled study. J Clin Oncol. 2005;23:8025-32.
36. McDonald BC, Conroy SK, Ahles TA, et al. Gray matter reduction associated with systemic chemotherapy for breast cancer: a prospective MRI study. Breast Cancer Res Treat. 2010;123:819-28.
37. McDonald BC, Conroy, SK, Ahles TA, et al. Alterations in brain activation during working memory processing associated with breast cancer and treatment: a prospective functional magnetic resonance imaging study. J Clin Oncol. 2012;30:2500-8.
38. Conroy SK, McDonald BC, Ahles TA, et al. Chemotherapy-induced amenorrhea: a prospective study of brain activation changes in neurocognitive correlates. Brain Imaging Behav. 2013;7:491-500.
39. Nelson CJ, Nandy N, Roth AJ. Chemotherapy and cognitive deficits: mechanisms, findings and potential interventions. Palliat Support Care. 2007;5:272-80.
40. Davis J, Ahlberg FM, Berk M, et al. Emerging pharmacotherapy for cancer patients with cognitive dysfunction. BMC Neurol. 2013;13:153.
41. Mar Fan HG, Clemons M, Xu W, et al. A randomised, placebo-controlled, double-blind trial of the effects of d-methylphenidate on fatigue and cognitive dysfunction in women undergoing adjuvant chemotherapy for breast cancer. Support Care Cancer. 2008;16:577-83.
42. Barton, DL, Burger K, Novotny PJ, et al. The use of Ginkgo biloba for the prevention of chemotherapy-related cognitive dysfunction in women receiving adjuvant treatment for breast cancer, N00C9. Support Care Cancer. 2013;21:1185-92.
43. Alvarez J, Fremonta LM, Granoff DL, Lundy A. The effect of EEG biofeedback on reducing postcancer cognitive impairment. Integr Cancer Ther. 2013;12:475-87.
44. Ferguson RJ, Ahles TA, Saykin AJ, et al. Cognitive-behavioral management of chemotherapy-related cognitive change. Psychooncology. 2007;16:772-7.
45. Ferguson RJ, McDonald BC, Rocque MA, et al. Development of CBT for chemotherapy-related cognitive change: results of a waitlist control trial. Psychooncology. 2012;12:176-86.
2 Commerce Drive
Cranbury, NJ 08512
All rights reserved.