Pancreatic Ca Vaccine Extends Survival After Surgery
An investigational therapeutic vaccine (GVAX immunotherapy) given with chemotherapy and radiation therapy following surgery for pancreatic cancer appears to improve survival for these difficult-to-treat patients
• ORLANDOAn investigational therapeutic vaccine (GVAX immunotherapy) given with chemotherapy and radiation therapy following surgery for pancreatic cancer appears to improve survival for these difficult-to-treat patients, according to the results of a phase II trial presented at the 2007 Gastrointestinal Cancers Symposium (abstract 106) (see image).
With a 3-year median follow-up, patients who received the vaccine had an overall median survival of 26.8 months, compared with about 20 months for historical controls, said Daniel A. Laheru, MD, assistant professor of medicine, Johns Hopkins Medical Institutions. Disease-free survival was 18.8 months.
The vaccine consists of lethally irradiated allogeneic pancreatic tumor cells engineered to secrete granulocyte macrophage colony-stimulating factor (GM-CSF). The GM-CSF molecule attracts immune cells to the vaccine site, where they encounter pancreatic cancer antigens. They then patrol the rest of the patient's body to destroy pancreatic tumor cells with the same antigen profile. Allogeneic cells were used because not enough tumor cells could be derived from each individual patient, Dr. Laheru said.
In a phase I dose-finding trial performed 6 years ago in 14 patients with pancreatic cancer who received the vaccine in addition to chemoradiotherapy following surgery, the vaccine was well tolerated, and there were three long-term survivors, all of whom received the highest dose, Dr. Laheru said.
The current phase II study involved 60 Johns Hopkins patients with operable disease: 53 patients were lymph node positive and 18 were margin positive.
All patients received their first vaccine injection 8 to 10 weeks after surgery. They subsequently were treated with fluorouracil-based chemotherapy and radiation therapy for 26 weeks. Patients who were disease free 1 month after completion of their chemoradiation then received three additional vaccine doses 1 month apart, followed by a fifth vaccine booster 6 months later.
The 1-year survival rate was 88%, and 2-year survival was 76%. These survival rates were compared to past studies of patients treated with surgery alone, which showed an average 1-year survival of 63% and a 2-year survival of 42%, Dr. Laheru said.
"The administration of gene-modified pancreas tumor cells is well tolerated," he said. Vaccine side effects were limited to local reactions at the injection site, which typically went away within a week to 10 days.
The researchers plan a follow-up study of the vaccine to include additional boosting with the best of adjuvant therapy. Dr. Laheru disclosed that he received research funding from Cell Genesys, Inc. South San Francisco, California, the company developing GVAX immunotherapy vaccines.
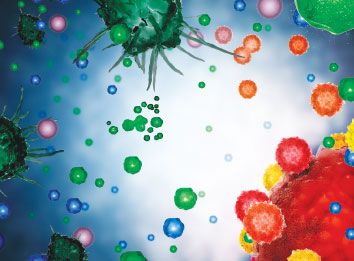
On the Cover
The image on page 1 is a stylized illustration of dendritic cells (DCs) that have been activated and matured by GM-CSF. The GVAX investigational vaccine, developed by Cell Genesys, Inc., is made from whole tumor cells that have been modified to secrete GM-CSF and then irradiated to prevent further cell division.
The theorized mechanism of action, based on preclinical data, involves DCs (depicted in grey) at the injection site becoming activated by the local secretion of GM-CSF and then taking up and processing antigens from the dying GVAX cells (blue/green) for presentation to T cells (red/yellow), which then migrate to the tumor where they can attack the tumor cells (lower right).