Pancreatic Cancer in the Older Patient
Pancreatic cancer is a disease seen predominantly in elderly patients. Compared to younger patients, older patients are more likely to present with early-stage disease and, therefore, may be candidates for aggressive local
ABSTRACT: Pancreatic cancer is a disease seen predominantly in elderly patients. Compared to younger patients, older patients are more likely to present with early-stage disease and, therefore, may be candidates for aggressive local treatment. Little published information exists on treatment outcomes for elderly patients with potentially resectable disease or those with locally advanced or metastatic pancreatic cancer. The limited information available suggests that elderly patients are as likely to benefit from surgery, radiation, and chemotherapy as younger patients. Despite this apparent benefit, elderly patients appear to have a worse long-term outcome. This may be due to the failure to offer them aggressive treatment or to comorbid conditions. Nevertheless, further studies need to be conducted in this area, and greater emphasis needs to be placed on including elderly patients in clinical trials. For elderly patients with terminal disease, there should be better use of palliative measures that may be of benefit. Each of these issues is discussed in detail. [ONCOLOGY 15(7):926-937, 2001]
Introduction
By 2030, 20% of the population will be over 65 years of age.[1] As the elderly population increases, oncologists will be faced with a progressively larger number of older patients with cancer. Pancreatic cancer is seen predominantly in older people, with incidence peaking from age 70 to 79 years.[2] According to the National Cancer Database, 68.5% of pancreatic cancers were diagnosed in those over age 65 years.[3] Overall, it is the fourth most common cause of cancer death, with an estimated 28,200 deaths in 2000.[4] However, this is mainly because it is the fourth most common cause of cancer death in patients over age 55 years.[4]
Older patients are more likely to have earlier-stage disease, but are less likely to undergo pancreatectomy.[3] A recent report showed that in those under age 55 years, 22.5% underwent pancreatectomy, compared to 13.5% in those aged 70 to 74 years, 10.7% in those aged 75 to 79, and 6.3% in those over age 80. Older patients are also less likely to receive chemotherapy than younger patients.[3] Although some of these patients are poor candidates for aggressive therapy, there is evidence from other tumor types that elderly patients do not receive appropriate treatment based on age alone.[5-8]
Despite the fact that the majority of patients with pancreatic cancer are elderly, there are very few data in the literature specific to this group of patients. Most of the existing data come from clinical trials, in which elderly patients are vastly underrepresented.[9,10] More research is needed to expand our knowledge of the treatment of pancreatic cancer in elderly patients, as the size and life expectancy of this population is ever increasing. This article will review the existing literature on the presentation and surgical and medical treatment of pancreatic cancer in elderly patients.
Characteristics of Pancreatic Cancer in the Elderly
TABLE 1

Disease-Specific 5-Year Survival of 44,438 Patients With Pancreatic Adenocarcinoma, 1985-1990
Several studies have shown that older patients are less likely to be staged than younger patients.[2,3] Among those who are staged, it appears that older patients present with earlier-stage disease. Despite this fact, older patients have a worse overall 5-year survival compared to younger patients (Table 1). This may be due to less aggressive treatment of elderly patients, as well as deaths due to comorbid conditions. However, no studies are available that adequately address this issue.
Kamisawa et al examined the pathologic features of pancreatic cancer in 89 elderly patients (> 70 years) and compared them to 184 younger patients.[11] With advancing age, proportionally more women were diagnosed. There were no differences in the grade or location (head/body/tail) of tumors in younger vs older patients, and there was no difference in the incidence of local spread. Elderly patients, however, did have fewer hematogenous metastases than younger patients.
In another study, it appeared that older patients had more diploid tumors, and younger patients had more aneuploid tumors (which may help explain the difference in hematogenous spread).[12]
Various events have been implicated in the pathogenesis of pancreatic cancer, such as K-ras mutation and p53 tumor-suppressor gene mutation. Sato et al studied the expression of p53 mutations in paraffinized tumors by using monoclonal antibodies to products of the p53 gene, DO-1-p53.[13] Overexpression of this product was significantly associated with a worse prognosis and was more common in older patients, independent of stage. The authors suggested that p53 mutations may be more common with aging. More research is needed to determine how aging is involved in the pathogenesis of pancreatic and other cancers.
Treatment Strategies
Surgery
The only potentially curative approach for pancreatic cancer is resection. Recent studies report 5-year survival rates ranging from 20% to 25% for those undergoing surgical resection.[2,14] Unfortunately, less than 20% of patients are considered resectable. With improvements in surgical techniques, the mortality for pancreaticoduodenectomy has diminished, and many centers are now reporting operative mortality rates of less than 5%. Although elderly patients present with earlier-stage disease, fewer of them undergo surgical treatment.[3]
A number of centers have reported their experience in elderly patients, and have shown that pancreatic resection can be performed in elderly patients without excess mortality, even in those over age 80 years. Fong et al compared the outcomes of patients under age 70 to those age 70 and older undergoing pancreatic resections.[15] Overall perioperative mortality was 6%. Although fewer patients over age 80 were scheduled for surgery, this group actually had fewer complications and no perioperative mortality. There was no difference in the length of hospital stay, complication rates, mortality rates, or rate of admission into the intensive care unit between age groups.
TABLE 2
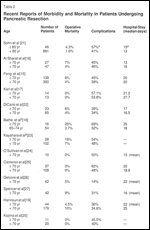
Recent Reports of Morbidity and Mortality in Patients Undergoing Pancreatic Resection
Univariate analysis showed that a history of cardiopulmonary disease, abnormal ECG, or abnormal chest x-ray preoperatively predicted for complications in elderly patients. Elderly patients did have a slightly lower median (18 vs 24 months) and 5-year survival (21% vs 29%). Other authors report similar survival rates for elderly patients compared to younger patients after surgery.[16-20] Morbidity and mortality data from recent surgical series in older patients are summarized in Table 2.[15-27]
Even less data are available for octogenarians. Sohn et al compared the outcomes of 46 patients over age 80 undergoing pancreaticoduodenectomy with 681 patients younger than 80.[21] Mortality was similar for the older and younger groups (4.3% vs 1.6%, respectively, P = NS), however, there were more postoperative complications and longer hospital stays among the older patients. Median survival for patients with pancreatic adenocarcinomas was no different between age groups (17 vs 18 months in the younger group). Elderly patients who undergo pancreaticoduodenectomy are a highly selected group; however, these studies show that this procedure can be performed without excess morbidity or mortality, and with a 5-year survival greater than 20%.
Combined-Modality Treatment in the Adjuvant Setting
For patients who have undergone surgical resection, radiation and chemotherapy are often given in an attempt to prolong survival. A Gastrointestinal Tumor Study Group (GITSG) trial showed an improvement in median survival in those treated with fluorouracil (5-FU) and radiation vs observation after resection (20 vs 11 months). The median age of patients in this study was 64 years for those treated and 59 years for controls. No specific toxicities or benefits of treatment were reported for older patients. However, age was not a significant prognostic factor for survival; only initial performance status and extent of tumor were predictive of survival.[28]
In another nonrandomized study, Yeo et al offered patients a choice between three adjuvant regimens: standard 5-FU/radiation, intensive 5-FU/radiation plus maintenance 5-FU, or no therapy. There were no differences between these groups in regard to sex, tumor characteristics, race, or intraoperative factors. However, patients receiving adjuvant therapy were younger than those observed. Patients who had longer postoperative stays and more complications were less likely to choose adjuvant therapy. It is not clear whether more of these patients were elderly. In multivariate analysis, only the size of the tumor, intraoperative blood loss, and use of adjuvant therapy were predictive of survival.[29] More recent studies report that 5-FU given as a prolonged intravenous infusion is better tolerated and allows for greater dose intensity than bolus 5-FU in patients with advanced pancreatic cancer.[30]
While most studies involving radiation include older patients, the specific toxicities and benefits of therapy for the elderly are not reported. Radiation therapy has been studied in elderly patients in other diseases, such as head and neck cancer, breast cancer, and prostate cancer.[31,32] In general, older patients tolerate radiation treatment as well as younger patients, although older patients being treated for bladder or rectal cancer may experience more toxicity.[32]
There are very few data, however, on tolerance of abdominal radiation in elderly patients. In general, the toxicities of abdominal radiation include nausea, vomiting, diarrhea, and anorexia.[33] Elderly patients may be more susceptible to dehydration, electrolyte disturbances, and infection, and may require multiple medications for symptom control while undergoing radiation therapy. Also, elderly patients often have problems with transportation, which makes completing therapy more difficult. Further investigations are needed to define the tolerance and benefit of radiation for pancreatic and other abdominal cancers in elderly patients.
Combined-Modality Treatment for Locally Advanced Disease
The combination of chemotherapy and radiation is often used for unresectable pancreatic cancer. The most common toxicities associated with the use of this combined modality therapy are nausea, vomiting, diarrhea, anorexia, and hematologic toxicity. Gastrointestinal bleeding has also been reported.[34-37] A GITSG trial established 5-FU and radiation as the treatment of choice, with improved survival over radiation alone.[34] The median age of patients in each of the groups in this study was 54 years and 61 years, respectively. In a multivariate analysis, age was not predictive of survival. The most common prognostic factors were good performance status (Eastern Cooperative Oncology Group [ECOG] 0 or 1), and pretreatment carcinoembryonic antigen (CEA) levels < 5.0 ng/mL. The most common toxicities were hematologic and nausea/vomiting. The rates of infection and mucocutaneous toxicity were quite low. However, there were no specific data on the tolerance of treatment in older patients.
A small study performed by ECOG randomized patients to 5-FU alone vs 5-FU and radiation. This study did not show a survival benefit with combined-modality therapy. Toxicity was more common in the combined-modality group, mostly leukopenia with sepsis. Again, there was no comment made on toxicity in older patients.[35] One phase I study did report toxicities in elderly patients receiving 5-FU/leucovorin and radiation for locally advanced pancreatic cancer.[38] Patients were treated with 40 Gy in 20 fractions (split course); 5-FU (300 to 375 mg/m2/d) and leucovorin (20 mg/m2/d) were given as a bolus on days 1 to 5 of radiation, and repeated the first 5 days of the second half of radiation. Of the 27 patients in the study, 4 were over age 70, and 11 were aged 55 to 69. Patients over age 70 and patients with poor performance status experienced more grade 3/4 toxicity. Two treatment-related deaths were reported (both infections)-a patient aged 44 years and a patient aged 80 years.
Recently, other radiosensitizing agents have been used with radiation in an attempt to improve on the results seen with 5-FU. Blackstock reported a phase I trial of twice-weekly gemcitabine (Gemzar) with concurrent radiation in patients with advanced disease.[39] The median age of patients was 61 years (range: 39 to 83 years). Again, there was no comment made on older patients’ tolerance of therapy. Dose-limiting toxicities were thrombocytopenia, neutropenia, and nausea/vomiting. Three patients achieved a partial response, and the other assessable 12 patients had stable local disease. Median survival was 11 months, and because of these favorable results, a phase II study is planned.
Paclitaxel (Taxol), another radiosensitizing agent, is being evaluated in the treatment of patients with unresectable pancreatic cancer. A recent phase II study of concurrent paclitaxel and radiation reported a 33% partial response rate, and 39% stable disease rate.[40] How the elderly patient population tolerates these new regimens awaits further study.
Chemotherapy for Advanced Disease
Numerous single-agent trials have failed to demonstrate any significant benefit for patients with advanced disease. Fluorouracil has long been the standard of care, with limited benefits in terms of survival or improved quality of life.[41] Combination chemotherapy, such as FAM (5-FU, doxorubicin [Adriamycin], mitomycin-C [Mutamycin]) and SMF (streptozocin [Zanosar], mitomycin-C, 5-FU), has not resulted in any significant improvement in survival.[41]
Gemcitabine
Gemcitabine, a pyrimidine antagonist, has recently been shown to confer both clinical benefit and a small improvement in survival compared to 5-FU. In a phase III study, 126 patients with unresectable pancreatic cancer were randomized to either weekly 5-FU at 600 mg/m2 or gemcitabine at 1,000 mg/m2/wk for 7 weeks, then every 3 of 4 weeks.[42] At study entry, most patients had impaired performance status and pain. The median age of enrollees was 61 to 62 years (range: 36 to 79 years). Clinical benefit, defined as improvement in performance status, narcotic requirements, and weight, was seen in 23.8% of patients receiving gemcitabine compared to 4.8% of those receiving 5-FU. Among those receiving gemcitabine, this response took an average of 7 weeks to attain, and lasted a mean of 18 weeks.
Most patients who received gemcitabine had stable disease, but three achieved a partial response (overall response rate: 5.4%). No responses were seen in the 5-FU group. The median survival was 5.65 months with gemcitabine and 4.41 months for those receiving 5-FU. Toxicities reported in both arms of the study were minimal. Grade 3/4 hematologic toxicity was observed in 26% of those receiving gemcitabine. Other common toxicities of gemcitabine included fever (grade 1/2, 30%), rash (grade 1/2, 24%), and nausea/vomiting (grade 3/4, 9.5%). The authors did not comment on any age-related differences in toxicity or clinical benefit.
Some small measure of clinical benefit with gemcitabine was also seen in a large compassionate-use protocol involving over 3,000 patients.[43] The median age of patients was 65 years, and the majority had metastatic disease. Patients were treated with doses as described in the Burris trial above. Median survival was 4.8 months, median time to progression was 2.7 months, and the overall response rate was 12%. Improvement in disease-related symptoms was measured by monitoring pain scores, analgesic use, and Karnofsky performance status. By the end of four cycles, 18.4% of patients had achieved improvements in disease-related symptoms. The authors did report that age had no effect on any patient outcomes.
Other studies using gemcitabine in elderly patients have confirmed its tolerability. It has been used extensively in the treatment of lung and bladder cancer.[44-47] The most common toxicities are mild hematologic toxicity, transient elevation in liver function tests, mild nausea/vomiting, rash, peripheral edema, and fever/flu-like symptoms.[48]
Two studies of gemcitabine in patients with lung cancer have specifically commented on age-related toxicities. In a group of 361 lung cancer patients receiving gemcitabine at 800 to 1,250 mg/m2/wk for 3 weeks in the setting of a clinical trial, there was no difference in grade 3/4 hematologic or nonhematologic toxicity between patients younger than age 65 and those age 65 and older.[49] Grade 3/4 neutropenia occurred in 25% and grade 3/4 thrombocytopenia in 1.9% of older patients. The most common grade 3/4 nonhematologic toxicity was nausea/vomiting, seen in 16% of older patients and 22% of younger patients. The authors also looked at dose delivery and found that elderly patients received dose reductions and omissions similar to younger patients.
Ricci et al reported a phase II trial of single-agent gemcitabine given at 1,000 mg/m2/wk for 3 weeks in 46 patients over age 70 years with advanced lung cancer.[50] Grade 3/4 neutropenia occurred in only 0.5% of patients, and no grade 3/4 thrombocytopenia was observed. The most common nonhematologic toxicity was a skin rash, seen in 4.3%. Patients over age 75 years received a median of three cycles, compared to four cycles in younger patients.
In a review of the safety of gemcitabine, Tonato et al reported on the toxicities seen in 790 patients enrolled in phase II studies using doses of 850 to 1,250 mg/m2/wk for 3 weeks, followed by 1 week of rest.[51] Patients over age 65 years did not experience excess toxicity, with the exception of more peripheral edema (25.3% vs 15.3%). Older patients actually had less nausea and vomiting. Grade 3/4 leukopenia was seen in only 8.6% of patients, and grade 3/4 thrombocytopenia in 4.7%, with age having no apparent effect. Martin et al reviewed the same database and found that the subset of patients over age 70 years did not experience any greater toxicity than those > 65 years old.[47]
Older patients may have more chronic renal insufficiency. Venook et al recently conducted a phase I trial of gemcitabine in patients with advanced cancer and either liver or renal dysfunction.[52] Median age in this study was between 64 and 66 years for each of the three cohorts studied. Gemcitabine was administered starting at 800 mg/m2/wk for 3 consecutive weeks of every 4-week cycle, with dose escalations as tolerated. The range of renal dysfunction seen included a creatinine level of 1.6 to 3.2 mg/dL; the range of hepatic dysfunction included a serum aspartate aminotransferase (AST) level of 37 to 530 U/L and total bilirubin from normal to 5.7 mg/dL.
The group with renal dysfunction experienced more skin toxicity, and the group with increased total bilirubin had transient hyperbilirubinemia. Interestingly, no hematologic toxicity was observed, and there were no differences in the clearance of gemcitabine among the patients. The authors recommended that patients with elevated bilirubin levels should be started at a reduced dose of gemcitabine (800 mg/m2/wk), whereas patients with elevated transaminases or renal dysfunction do not need an initial dose reduction.
Novel Agents
Other forms of chemotherapy, as well as novel agents, are being assessed in an ongoing series of phase II and III trials. Several trials are attempting to enhance the activity of gemcitabine with other drugs such as oxaliplatin or novel agents such as pemetrexed (Alimta) and ISIS 2503. A phase III trial is currently evaluating the activity of 9-nitrocamptocin (RFS2000) compared to gemcitabine. Each of these trials is designed to include patients of all ages. However, in general, no assessment of tolerability by age groups is included as part of the primary analysis of these trials.
Palliation
Because most patients with pancreatic cancer present with incurable locally advanced or metastatic disease, palliative care is important in their management. Most patients require pain control, and many will need treatment for jaundice and duodenal/gastric obstruction.
In general, elderly patients with cancer pain are undertreated,[53,54] and many of these patients underreport their pain.[54-56] Also, there are misconceptions about an older patient’s tolerance of pain and ability to use opioids safely.[54,55] Because the majority (> 80%) of patients with pancreatic cancer suffer from pain, frequent assessment and treatment of pain is extremely important. Untreated pain leads to a decreased quality of life, depression, diminished performance status, and sleep disturbances.[57,58]
In many cases, pain can be treated effectively with oral narcotics, but in treating elderly patients with narcotics, a few points need to be emphasized. Older patients are often more sensitive to opioids, and may require lower initial dosing and longer intervals between dosing.[54,55,59] Elderly patients often receive multiple medications that may exacerbate side effects, such as mental status or behavioral changes. Also, patients with renal impairment have decreased clearance of morphine, and require careful dosing and monitoring.[54]
Certain opioids are not recommended for older patients. These include meperidine, methadone, and propoxyphene. Elderly patients, especially those with impaired renal function, may have more side effects with these drugs due to prolonged half-life or accumulation of metabolites. Transdermal fentanyl (Duragesic) should also be used with caution, especially in those with poor fat stores, muscle wasting, or abnormal liver or kidney function.[55] This drug should be avoided in opioid-naive patients. Elderly patients can be treated successfully with narcotics, but require more careful dosing and frequent assessments of their pain.
Celiac Block for Pain Control
Another option available for control of pain is celiac plexus block, performed either at the time of laparotomy or percutaneously. In a randomized study of alcohol splanchnicectomy vs placebo in patients undergoing exploration for pancreatic cancer, those receiving a block had no increase in mortality or morbidity, but did report lower pain scores for up to 6 months.[60] The mean age of patients in this study was 64 years. A percutaneous celiac block can also be used, and is associated with success rates of 80% to 90%.[61,62-64] Many patients experience pain relief for up to 6 months,[63] thus decreasing the amount of narcotics they use, and diminishing the side effects of these drugs.
Side effects of the procedure include hypotension, increased bowel motility, spread of the neurolytic solution to adjacent structures, and perforation of other organs. Paralysis has rarely been reported.[62,64] Elderly patients with comorbid diseases may be more susceptible to a hypotensive episode. However, this procedure has very little overall morbidity, and should be considered in patients with pain related to their pancreatic cancer.
Palliation of Biliary and Duodenal Obstruction
Obstructive jaundice is seen in up to 70% of newly diagnosed patients. Untreated, patients will suffer from liver failure, anorexia, nausea, malnutrition, and often intractable pruritus.[61,63] Biliary obstruction can be palliated surgically or with stent placement. Most patients are treated successfully with an endoscopically placed stent, with resolution of obstruction in 90% of patients.[61] Stent placement has been shown to improve symptoms of obstruction, as well as quality of life.[65,66]
Complications occur in roughly 20% of patients, but procedure-related mortality is low (1.3%).[63] Complications of stent placement include infection, pancreatitis, duodenal ulceration, stent dislocation, and occlusion of the stent.[67] Older age was found to be an unfavorable prognostic factor for stent patency in one study that used plastic stents.[68] Newer metal expandable stents provide longer patency (> 6 months) and are associated with fewer complications than plastic stents.[67,69-71] If these stents become occluded, another stent can be placed or a variety of cleaning procedures may open the stent.
For patients in whom stent placement is unsuccessful, percutaneous stent placement is an option. This can be internalized in most patients. Surgical biliary bypass is usually a last option for patients in whom stent placement is unsuccessful. A cost-effective analysis showed that endoscopic stenting provides equivalent survival with lower costs and shorter hospital stays when compared to surgical palliation.[72]
Duodenal obstruction occurs in about 20% of patients who do not undergo initial gastric bypass. Patients may be treated with prophylactic gastrojejunostomy at the time of initial laparotomy without increased mortality. For those who develop gastric outlet obstruction, palliative gastric bypass can be performed. Metal stents have also recently been used with success in palliating malignant gastric outlet obstruction.[73,74] Elderly patients with reasonable life expectancy should be offered these palliative procedures.
Conclusions
Oncologists will be faced with treating an increasing number of older patients with pancreatic cancer. Older patients present with earlier-stage disease, and may have less hematogenous metastasis than younger patients. In older patients who are surgical candidates, a pancreaticoduodenectomy can be performed without undue morbidity or mortality. Adjuvant chemotherapy with concurrent radiation should also be considered in older patients. However, there are very few data on the tolerability of combined-modality treatment in older patients in either the adjuvant or advanced setting. More research needs to be conducted in this area.
Palliative chemotherapy, specifically gemcitabine, can be administered safely to elderly patients. It is well tolerated and provides temporary palliation for some patients with advanced disease. Initial dose reductions are not needed based on age alone, but may be required for those with elevated bilirubin levels. Future studies utilizing newer agents should include older patients, and the elderly should be encouraged to participate in clinical trials whenever possible. Supportive care is extremely important in patients with advanced disease. Pain control can be accomplished safely with careful administration of narcotics. Celiac plexus blockade can also be extremely helpful in pain control. Obstructive jaundice can usually be managed successfully with endoscopic stent placement, and gastric outlet obstruction with either stenting or surgical bypass. There is a paucity of data regarding the tolerance of these palliative procedures in older patients, and more research efforts are needed to improve the care of the elderly.
References:
1. US Bureau of the Census: Current Population Reports, specialstudies, P23-190, 65+ in the United States. Washington, DC, US GovernmentPrinting Office, 1996.
2. Sener SF, Fremgen A, Menck HR, et al: Pancreatic cancer: Areport of treatment and survival trends for 100,313 patients diagnosed from1985-1995, using the National Cancer Database. J Am Coll Surg 189:1-7, 1999.
3. Niederhuber JE, Brennan MF, Menck HR: The National CancerDatabase report on pancreatic cancer. Cancer 76:1671-1676, 1995.
4. Greenlee RT, Murray T, Bolden S, et al: Cancer statistics,2000. CA Cancer J Clin 50:7-33, 2000.
5. Greenfield S, Blanco DM, Elashoff RM, et al: Patterns of carerelated to age of breast cancer patients. JAMA 257:2766-2770, 1987.
6. Samet J, Hunt MC, Key C, et al: Choice of cancer treatmentvaries with age of patient. JAMA 255:3385-3390, 1986.
7. Goodwin JS, Hunt WC, Samet JM: Determinants of cancer therapyin elderly patients. Cancer 72:594-601, 1993.
8. Mor V, Masterson-Allen S, Goldberg RJ, et al: Relationshipbetween age at diagnosis and treatments received by cancer patients. J AmGeriatr Soc 33:585-589, 1985.
9. Hutchins LF, Unger JM, Crowley JJ, et al: Underrepresentationof patients 65 years of age or older in cancer treatment trials. N Engl J Med341:2061-2067, 1999.
10. Goodwin JS, Hunt WC, Humble CG, et al: Cancer treatmentprotocols: Who gets chosen? Arch Intern Med 148:2258-2260, 1988.
11. Kamisawa T, Yuyang T, Egawa N, et al: Characteristics ofpancreatic carcinoma in the elderly. Int J Pancreatol 24:31-34, 1998.
12. Hatori T: A clinicopathologic study of ductal adenocarcinomaof the head of the pancreas in aged patients 70 years and older. J Jpn Panc Soc8:506-515, 1993.
13. Sato Y, Nio Y, Song M, et al: p53 protein expression asprognostic factor in human pancreatic cancer. Anticancer Res 17:2779-2788, 1997.
14. Yeo CJ, Cameron JL, Lillemoe KD, et al:Pancreaticoduodenectomy for cancer of the head of the pancreas: 201 patients.Ann Surg 221:721-733, 1995.
15. Fong Y, Blumgart LH, Fortner JG, et al: Pancreatic or liverresection for malignancy is safe and effective for the elderly. Ann Surg222:426-437, 1995.
16. Al Sharaf K, Andren-Sandberg A, Ihse I: Subtotalpancreatectomy for cancer can be safe in the elderly. Eur J Surg 165:230-235,1999.
17. Karl RC, Smith SK, Fabri PJ: Validity of major canceroperations in elderly patients. Ann Surg Oncol 2:107-113, 1995.
18. Bathe OF, Levi D, Caldera H, et al: Radical resection ofperiampullary tumors in the elderly: Evaluation of long-term results. World JSurg 24:353-358, 2000.
19. Hannoun L, Christophe M, Ribeiro J, et al: A report of 44instances of pancreaticoduodenal resection in patients more than 70 years ofage. Surg Gynecol Obstet 177:556-560, 1993.
20. Kojima Y, Yasukawa H, Katayama K, et al: Postoperativecomplications and survival after pancreaticoduodenectomy in patients aged over70 years. Surg Today 22:401-404, 1992.
21. Sohn TA, Yeo CJ, Cameron JL, et al: Shouldpancreaticoduodenectomy be performed in octogenarians? J Gastrointesterol Surg2:207-216, 1998.
22. DiCarlo V, Balzano G, Zerbi A, et al: Pancreatic cancerresection in elderly patients. Br J Surg 85:607-610, 1998.
23. Kayahara M, Nagakawa T, Ueno K, et al: Pancreatic resectionfor periampullary carcinoma in the elderly. Surg Today 24:229-233, 1994.
24. O’Sullivan KL, Hart MJ: Pancreaticoduodenectomy in theelderly: A ten-year experience. Contemp Surg 48:265-269, 1996.
25. Cameron JL, Pitt, HA, Yeo CJ, et al: One hundred andforty-five consecutive pancreaticoduodenectomies without mortality. Ann Surg217:430-438, 1993.
26. Delcore R, Thomas JH, Hermreck AS: Pancreaticoduodenectomyfor malignant pancreatic and periampullary neoplasms in elderly patients. Am JSurg 162:532-546, 1991.
27. Spencer MP, Sarr MG, Nagorney DM: Radical pancreactectomyfor pancreatic cancer in the elderly. Is it safe and justified? Ann Surg212:140-143, 1990.
28. Kalser MH, Ellenberg SS: Pancreatic cancer. Adjuvantcombined radiation and chemotherapy following curative resection. Arch Surg120:899-903, 1985.
29. Yeo CJ, Abrams RA, Grochow LB, et al:Pancreaticoduodenectomy for pancreatic adenocarcinoma: Postoperative adjuvantchemoradiation improves survival. Ann Surg 225:621-636, 1997.
30. Poen JC, Collins HL, Niederhuber JE, et al:Chemoradiotherapy for localized pancreatic cancer: Increased dose intensity andreduced acute toxicity with concomitant radiotherapy and protracted venousinfusion 5-fluorouracil. Int J Radiat Oncol Biol Phys 40:93-99, 1998.
31. Wasil T, Lichtman SM, Gupta V, et al: Radiation therapy incancer patients 80 years of age and older. Am J Clin Oncol 23:526-530, 2000.
32. Olmi P, Cefaro GA, Balzi M, et al: Radiotherapy in the aged.Clin Geriat Med 13:143-168, 1997.
33. Ahlgren JD: Gastrointestinal cancer in the elderly.Gastroenterol 15:627-639, 1999.
34. Moertel CG, Frytak S, Hahn RG, et al: Therapy of locallyunresectable pancreatic carcinoma: A randomized comparison of high-dose (6000rads) radiation alone, moderate-dose radiation (4000 rads + 5-fluorouracil), andhigh-dose radiation + 5-fluorouracil. Cancer 48:1705-1710, 1981.
35. Klaassen DJ, MacIntyre JM, Cotton GE, et al: Treatment oflocally unresectable cancer of the stomach and pancreas: A randomized comparisonof 5-fluorouracil alone with radiation plus concurrent and maintenance5-fluorouracil-An ECOG study. J Clin Oncol 3:373-378, 1985.
36. Moertel CG, Gunderson LL, Mailliard JA, et al: Earlyevaluation of combined fluorouracil and leucovorin as a radiation enhancer forlocally unresectable, residual, or recurrent gastrointestinal carcinoma. J ClinOncol 12:21-27, 1994.
37. Dobelbower RR, Borgelt BB, Strubler KA, et al: Precisionradiotherapy for cancer of the pancreas: Technique and results. Int J RadiatOncol Biol Phys 6:1127-1133, 1980.
38. Hsue V, Wong CS, Moore M, et al: A phase I study of combinedradiation therapy with 5-fluorouracil and low-dose folinic acid in patients withlocally advanced pancreatic or biliary carcinoma. Int J Radiat Oncol Biol Phys34:445-450, 1996.
39. Blackstock AW, Bernard SA, Richards F, et al: Phase I trialof twice-weekly gemcitabine and concurrent radiation in patients with advancedpancreatic cancer. J Clin Oncol 17:2208-2212, 1999.
40. Safran H, Akerman P, Cioffi W, et al: Paclitaxel andconcurrent radiation therapy for locally advanced adenocarcinomas of thepancreas, stomach, and gastroesophageal junction. Semin Radiat Oncol 9(suppl1):53-57, 1999.
41. Schnall SF, Macdonald JS: Chemotherapy of adenocarcinoma ofthe pancreas. Semin Oncol 23:220-228, 1996.
42. Burris HA, Moore MJ, Andersen J, et al: Improvements insurvival and clinical benefit with gemcitabine as first-line therapy forpatients with advanced pancreas cancer: A randomized trial. J Clin Oncol15:2403-2413, 1997.
43. Storniolo AM, Enas NH, Brown CA, et al: An investigationalnew drug treatment program for patients with gemcitabine. Cancer 85:1261-1268,1999.
44. Shepherd FA: Phase II trials of single-agent activity ofgemcitabine in patients with advanced non-small-cell lung cancer: An overview.Anticancer Drugs 6 (suppl) 6:19-25, 1995.
45. Stadler WM, Kuzel T, Roth B, et al: Phase II study ofsingle-agent gemcitabine in previously untreated patients with metastaticurothelial cancer. J Clin Oncol 15:3394-3398, 1997.
46. Moore MJ, Tannock IF, Ernst DS, et al: Gemcitabine: Apromising new agent in the treatment of advanced urothelial cancer. J Clin Oncol15:3441-3445, 1997.
47. Martin C, Ardizonni A, Rosso R: Gemcitabine: Safety profileand efficacy in non-small cell lung cancer unaffected by age. Aging 9:297-303,1997.
48. Green MR: Gemcitabine safety overview. Semin Oncol 23 (5suppl 10):32-35, 1996.
49. Shepherd FA, Abratt RP, Anderson H, et al: Gemcitabine inthe treatment of elderly patients with advanced non-small cell lung cancer.Semin Oncol 24 (2 suppl 7): 50-55, 1997.
50. Ricci S, Antonuzzo A, Galli L, et al: Gemcitabinemonotherapy in elderly patients with advanced non-small cell lung cancer: Amulticenter phase II study. Lung Cancer 27:75-80, 2000.
51. Tonato M, Mosconi AM, Martin C: Safety profile ofgemcitabine. Anticancer Drugs 6(suppl 6):27-32, 1995.
52. Venook AP, Egorin MJ, Rosner GL, et al: Phase I andpharmacokinetic trial of gemcitabine in patients with hepatic or renaldysfunction: Cancer and Leukemia Group B 9565. J Clin Oncol 18:2780-2787, 2000.
53. Bernabei R, Gambassi G, Lapane K, et al: Management of painin elderly patients with cancer. JAMA 279:1877-1882, 1998.
54. Sheehan DC, Forman WB: Symptomatic management of the olderperson with cancer. Clin Geriatr Med 13:203-219, 1997.
55. Ferrell BR, Ferrell BA: Pain in elderly persons, in McGuireDB, Yarbro CH, Ferrell BR (eds): Cancer Pain Management, pp 273-287. Boston,Jones and Bartlett Publishers, 1995.
56. Brescia FJ, Adler D, Gray G, et al: Hospitalized advancedcancer patients: A profile. J Pain Symptom Manage 5:221-227, 1990.
57. Ferrell BA, Ferrell BR, Osterweil D: Pain in the nursinghome. J Am Geriatr Soc 38:409-414, 1990.
58. Ferrell BR, Wisdom C, Wenzl C: Quality of life as an outcomevariable in the management of cancer pain. Cancer 63:2321-2327, 1989.
59. Kaiko RF, Wallenstein SL, Rogers AG, et al: Narcotics in theelderly. Med Clin North Am 66:1079-1089, 1982.
60. Lilliemoe KD, Cameron JL, Kaufman HS, et al: Chemicalsplanchnicectomy in patients with unresectable pancreatic cancer. A prospectiverandomized trial. Ann Surg 217:447-457, 1993.
61. Lillimoe KD, Pitt HA: Palliation, surgical and otherwise.Cancer 78:605-614, 1996.
62. Thompson GE, Moore DC, Bridenbaugh LD, et al: Abdominal painand alcohol celiac plexus nerve block. Anesth Analg 56:1-5, 1977.
63. Russell RCG: Palliation of pain and jaundice: An overview.Ann Oncol 10(suppl 4):165-169, 1999.
64. Caraceni A, Portenoy RK: Pain management in patients withpancreatic carcinoma. Cancer 78:639-653,1996.
65. Ballinger AB, McHugh M, Catnach SM, et al: Symptom reliefand quality of life after stenting for malignant bile duct obstruction. Gut35:467-470, 1994.
66. Luman W, Cull A, Palmer KR: Quality of life in patientsstented for malignant biliary obstruction. Eur J Gastroenterol Hepatol9:481-484, 1997.
67. Schofl R, Brownstone E, Reichel W, et al: Malignantbile-duct obstruction: Experience with self-expanding metal endoprostheses(Wallstents) in Austria. Endoscopy 26:592-596, 1994.
68. Matsuda Y, Shimakura K, Akamatsu T: Factors affecting thepatency of stents in malignant biliary obstructive disease: Univariate andmultivariate analysis. Am J Gastroenterol 86:843-849, 1991.
69. O’Brien S, Hatfield AR, Craig PI, et al: A 3-year followup of self expanding metal stents in the endoscopic palliation of long-termsurvivors with malignant biliary obstruction. Gut 36:618-621, 1995.
70. Neuhaus H, Hagenmuller F, Griebel M, et al: Percutaneouscholangioscopic or transpapillary insertion of self-expanding biliary metalstents. Gastrointest Endosc 37:31-37, 1991.
71. Knyrim K, Wagner HJ, Pausch J, et al: A prospective,randomized, controlled trial of metal stents for malignant obstruction of thecommon bile duct. Endoscopy 25:207-212, 1993.
72. Raikar GV, Melin MM, Ress A, et al: Cost-effective analysisof surgical palliation versus endoscopic stenting in the management ofunresectable pancreatic cancer. Ann Surg Oncol 3:470-475, 1996.
73. Venu RP, Pastika BJ, Kini M, et al: Self-expandable metalstents for malignant gastric outlet obstruction: A modified technique. Endoscopy30:553-558, 1998.
74. Feretis C, Benakis P, Dimopoulos C, et al: Palliation ofmalignant gastric outlet obstruction with self-expanding metal stents. Endoscopy28:225-228, 1996.