PARP Inhibition in Epithelial Ovarian Cancer: High Hopes Undergo a Reality Check
This article reviews the trials that have been conducted with PARP inhibitors in epithelial ovarian cancer, fallopian tube cancer, and primary peritoneal cancer, and places the impact of those results in the larger context of PARP inhibitor development.
The treatment strategy of poly(ADP-ribose) polymerase (PARP) inhibition capitalizes on the inherent defect in homologous recombination that occurs in BRCA-deficient tumors by inhibiting the alternative DNA repair pathway involving base excision repair. Although PARP inhibitors were initially considered a potential treatment specifically for tumors with germline BRCA mutations, evidence of frequent somatic deficiency in the BRCA pathway has caused reconsideration of that approach. PARP inhibitors have been shown to have activity in epithelial ovarian, fallopian tube, and primary peritoneal cancer in phase I and II clinical trials. Responses have been seen in both BRCA-deficient and sporadic tumors, and they do not appear to require platinum sensitivity. Although PARP inhibitors are well tolerated as monotherapy, additional study is required to determine their efficacy and toxicity in combination with chemotherapy and other targeted agents. Many hurdles remain along the pathway to drug registration, but the motivation of the community of ovarian cancer patients, researchers, and clinicians to find new treatments may speed the process.
The identification of BRCA1 and BRCA2 in the early 1990s as genes that, when mutated, are associated with an increased risk of breast, epithelial ovarian, fallopian tube, primary peritoneal, and other cancers opened a new frontier in managing hereditary cancer risk. The ability to delineate a high-risk subset of patients was of particular benefit to research into epithelial ovarian cancer (EOC), given its poor prognosis, frequent late stage at diagnosis, and relative lack of effective screening techniques. Further study has documented that, depending on the population evaluated, 5% to 15% of EOC cases are hereditary in origin. Mutations in BRCA1/2 or the mismatch repair mutations of Lynch syndrome account for almost all of that risk, although the great majority of EOC cases are sporadic in origin.
Groups at high risk for a disease provide ideal populations for research given their relatively high event rate, which makes studies shorter, smaller, and less expensive to conduct than when the general population is evaluated. These patients also tend to be highly motivated to participate in research, since the results may benefit not only their own treatment but also the care of their family members. In light of the desperate need for improvement in the treatment of EOC, development of poly(ADP-ribose) polymerase (PARP) inhibitors in the treatment of BRCA-deficient cancer has been closely monitored by patients, as well as by clinicians and researchers. The great hope is that lessons learned from the high-risk population will lead to insights into sporadic EOC.
This article will review the trials that have been conducted with PARP inhibitors in EOC, fallopian tube cancer (FTC), and primary peritoneal cancer (PPC). The impact of those results will then be placed in the larger context of PARP inhibitor development.
PARP Inhibitor Trials in Ovarian Cancer
The early history of PARP inhibition has been chronicled thoroughly, including in reviews by Comen et al[1] and Rios et al,[2] and will not be discussed in depth here. The strategy capitalizes on the inherent defect in homologous recombination that occurs in BRCA-deficient tumors by inhibiting the alternative DNA repair pathway involving base excision repair. Base excision repair relies on PARP. If that pathway is blocked via PARP inhibition, the loss of both repair mechanisms leads to accumulation of DNA breaks and, ultimately, cell death. The concept of synthetic lethality, originally described in 1946, has gained new life in the setting of these two DNA repair defects, which individually are not lethal but become so when combined.[3]
TABLE 1

Completed Clinical Trials of PARP Inhibitors in Epithelial Ovarian Cancer
Tables 1 and 2 include the trials listed on www.ClinicalTrials.gov that evaluate PARP inhibition in EOC and other gynecologic cancers. The list was synthesized by searching for “PARP inhibitors AND ovarian cancer” and conducting separate searches for each of the PARP inhibitors currently in development (AG014699 [PF-01367338], olaparib [KU59436, AZD2281], veliparib [ABT-888], iniparib [BSI-201], INO-1001, GP121016, CEP-9722, MK4827, and BMN-673). Of these agents, results for trials in EOC were found for AG014699, olaparib, veliparib, iniparib, and MK4827.
Although PARP inhibitors were initially considered a potential treatment specifically for tumors with germline BRCA mutations, evidence of frequent somatic deficiency in the BRCA pathway has led to reconsideration of that approach. In an analysis of 235 EOC cases, Hennessy et al noted that among 44 BRCA1/2 mutations detected, at least 43% of the BRCA1 mutations and 29% of the BRCA2 mutations were somatic in nature. (Not all patients had germline DNA available for testing.)[4] In addition, the Cancer Genome Atlas analysis of serous EOC recently documented that a combination of germline and somatic events led to mutations in BRCA1/2 in 22% of cases, but that the frequency of other mutations in EMSY, PTEN, RAD51C, ATM, ATR, and Fanconi anemia genes suggests that approximately 50% of serous EOC cases have disruption of the homologous recombination pathway and may be susceptible to PARP inhibitor therapy.[5] The shift towards evaluating PARP inhibitor activity in broader clinical populations than BRCA-deficient EOC, FTC, and PPC is illustrated in Tables 1 and 2; the completed trials shown in Table 1 all focused on populations with germline mutations, whereas 21 of 26 ongoing trials listed in Table 2 have a sporadic component.
Completed trials
TABLE 2
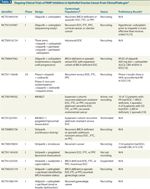
Ongoing Clinical Trials of PARP Inhibitors in Epithelial Ovarian Cancer From ClinicalTrials.gov
The trials included in Table 1 are listed as “completed” on www.ClinicalTrials.gov and/or have results published in manuscript format. Fong et al described the earliest experience with PARP inhibitor treatment in EOC in a phase I trial with dose escalation of olaparib from 10 mg daily to 600 mg twice daily in a population of patients with recurrent solid tumors.[6] The maximum tolerated dose was 400 mg twice daily. Interestingly, PARP inhibition as measured in peripheral mononuclear cells exceeded 90% after a dose of 60 mg twice daily, although clinical responses were not seen until the 100-mg twice-daily dose level. Patients with BRCA-deficient cancer (eight with EOC and one with breast cancer) accounted for all of the partial or complete radiologic responses seen. This observation led to an expansion cohort in the BRCA-deficient population.[7] A dose of 200 mg twice daily was chosen due to concerns about possible increased toxicity in mutation carriers. However, no increase in side effects was seen; olaparib was well tolerated, with the most common complaints being fatigue and gastrointestinal symptoms. The clinical benefit rate was 46% in the expansion cohort, with a median response duration of 28 weeks. A statistically significant increase in response rate was seen among platinum-sensitive vs platinum-resistant EOC patients, leading to questions about whether platinum sensitivity is a surrogate for predicting response to PARP inhibition and whether patients with platinum-resistant tumors should be excluded in future studies.
A subsequent phase II trial of olaparib was performed as an international collaboration known as ICEBERG (International Collaborative Expertise for BRCA Education and Research through Genetics) 2.[8] The study population included women with recurrent, BRCA-deficient EOC, FTC, or PPC. Two dose cohorts were included: a 100-mg twice-daily group (the dose at which responses were first seen in the phase I trial) and a 400-mg twice-daily group (the maximum tolerated dose in the phase I trial). The primary objective of overall response rate varied from 13% in the low-dose group to 33% in the high-dose group. As in the phase I trial, olaparib was well tolerated, with fatigue, nausea, and anemia as the most common toxicities.
Another phase II trial with olaparib was performed in Canada and included women with recurrent, high-grade serous or undifferentiated sporadic EOC; BRCA-deficient EOC, FTC, or PPC; or triple-negative breast cancer.[9] Among 91 patients enrolled, 64 with EOC were treated. As expected, most of these patients had sporadic disease (47 of 64); among the 17 mutation carriers, 65% had BRCA1 mutations, while 29% had BRCA2 mutations and 1 patient had both. The primary endpoint of objective response rate was 41% in mutation carriers and 24% in sporadic EOC patients, a marked difference from the dose-escalation portion of the phase I trial, where no responses were seen in non–mutation carriers. Interestingly, no responses were seen in 26 breast cancer patients, even among the 10 patients with germline mutations.
The only other completed trial of a PARP inhibitor including EOC patients is a phase I study combining veliparib with temozolomide (Temodar). The combination was of interest due to preclinical evidence of synergy.[10,11] No final results for the phase I study have been reported to date, although a follow-up phase II trial has completed accrual.
Ongoing trials
Table 2 lists the 26 trials that were identified in ClinicalTrials.gov as ongoing, with 14 in phase I, 2 in phase I/II, and 10 in phase II. No phase III trials are in progress currently. Nine trials are active but have completed accrual; two others either have been suspended or terminated but were included in this table because final results have not been published in manuscript format. Preliminary results presented as abstracts appear in the last column of Table 2 and are summarized below.
Monotherapy trials of olaparib, veliparib, iniparib, MK4827, and AG014699 are included, of which only the veliparib study continues to enroll patients. Preliminary results from a phase I study of MK4827 show that 10 of 12 partial responses seen were in EOC patients, with a mix of BRCA-deficient (7) and sporadic (3) cases.[12] In addition, four of eight patients with stable disease had EOC, evenly split between BRCA-deficient and sporadic tumors. Another monotherapy trial of AG014699 in a phase II setting included women with BRCA-deficient EOC and/or breast cancer.[13] Of 41 patients enrolled, 24 had EOC. The overall response rate for the entire cohort was quite low at 5%, but stable disease contributed to a clinical benefit rate of 32%.
Finally, two randomized phase II trials incorporating olaparib monotherapy have been reported. In the first, women with recurrent, BRCA-deficient EOC were randomized between olaparib at 200 mg twice daily, olaparib at 400 mg twice daily, and pegylated liposomal doxorubicin (PLD).[14] Initial results show median progression-free survival times of 6.5 months, 8.8 months, and 7.1 months, respectively, for the three treatment groups. The highest rate of confirmed response was in the high-dose olaparib group (31%). In the second randomized phase II trial, olaparib at 400 mg twice daily was compared with placebo in a cohort of women with recurrent, platinum-sensitive, serous EOC as maintenance therapy after partial or complete response to platinum therapy.[15] Preliminary findings include an improvement in progression-free survival from 4.8 to 8.4 months, which was statistically significant. The data were not mature enough to allow an analysis of overall survival.
It is evident from the remaining trials listed that combinations of a PARP inhibitor with traditional chemotherapy and/or other targeted agents are of interest. Chemotherapy agents under study in combination with various PARP inhibitors include carboplatin, paclitaxel, irinotecan, carboplatin/pac-litaxel, carboplatin/gemcitabine, PLD, and topotecan. The fundamental question of drug sequence is being investigated in a phase I study of olaparib and carboplatin.[16] Preclinical evidence suggests that administering olaparib before carboplatin diminishes DNA damage, possibly because PARP inhibition preceding cytotoxic chemotherapy enhances alternative DNA repair pathways and recovery from chemotherapy.[17] Preliminary results from another phase I study of olaparib and carboplatin with maintenance olaparib until progression include a recommended phase II dose of olaparib 400 mg twice daily and carboplatin AUC5, as well as an impressive 83% clinical benefit rate in patients with recurrent EOC.[18]
A phase I study of veliparib and irinotecan in recurrent malignancies is evaluating escalating doses of veliparib given twice daily for 15 days out of a 21-day cycle while irinotecan is held at a fixed dose of 100 mg/m2 on days 1 and 8.[19] Preliminary results include a clinical benefit rate of 61% in 32 patients, 7 of whom have EOC. Dose-limiting toxicities include fatigue, diarrhea, febrile neutropenia, and bone-marrow suppression. The recommended phase II dose is 40 mg of veliparib twice daily for 15 days on/6 days off, with irinotecan at a dose of 100 mg/m2 intravenously on days 1 and 8.
Single-arm phase II trials of iniparib with gemcitabine and carboplatin in platinum-sensitive and platinum-resistant recurrent EOC yielded preliminary results recently.[20,21] In the platinum-sensitive group, 12 of 17 women had a confirmed response, while 6 of 19 had a confirmed response in the platinum-resistant group.
PARP inhibitors are also being studied in combination with the antiangiogenic agents bevacizumab (Avastin) and cediranib (AZD2171). Veliparib is being evaluated along with carboplatin, paclitaxel, and bevacizumab in the Gynecologic Oncology Group phase I trial 9923. This is the only trial of frontline treatment of EOC, FTC, or PPC now in progress. Cediranib is being evaluated with olaparib in a phase I/II trial enrolling patients with recurrent EOC, FTC, or PPC; preliminary results from the phase I component include a 56% unconfirmed response rate, without unexpected toxicity.[22]
Issues Regarding Future Trials
The clinical trials conducted with PARP inhibitors in EOC have taught us many lessons, but they raise even more questions. What began as a very rational drug development has evolved into an increasingly complex path to drug approval.
1. Multiple PARP inhibitors appear to be active in EOC in phase I and II trials. Currently, there are no data comparing one PARP inhibitor to another in the clinical arena. In contrast to what was previously thought, however, recent data from cell lines suggest that iniparib does not share the same mechanism of action as olaparib and veliparib.[23] Although all three drugs create γH2AX foci, olaparib and veliparib do so via inhibition of PARP1 and PARP2, while iniparib likely inhibits PARP5 and PARP6. The clinical implications of this distinction are unclear. History has taught us that many targeted agents are not as targeted in practice as they seemed during their initial development and that sometimes the actual targets differ substantially from the theoretical ones.
2. The toxicities associated with PARP inhibitors as monotherapy are generally mild, especially when compared with traditional chemotherapy. This has prompted consideration of their use in the chemoprevention setting. The population of BRCA mutation carriers unaffected by cancer will continue to grow as patients, their families, and healthcare providers become increasingly aware of hereditary cancer syndromes. The Australian Ovarian Cancer Study recently documented germline BRCA1/2 mutations in 13% of the patients, but only 57% of the mutation carriers had pedigrees suggestive of a hereditary syndrome.[24] Indeed, the National Comprehensive Cancer Network guidelines now include the recommendation that a diagnosis of EOC, FTC, or PPC generates a referral for genetic counseling and testing, regardless of a woman's family history (www.nccn.org, version 1.2011).
Given the lack of an effective screening strategy for EOC, chemoprevention would be an attractive alternative to the current reliance on risk-reducing salpingo-oophorectomy in BRCA mutation carriers. However, the amount of toxicity from preventive therapy that a young, otherwise healthy woman in her reproductive years can tolerate differs radically from what can be tolerated by an older woman with life-threatening cancer. Data on the effects of long-term exposure to PARP inhibition, especially with respect to potential induction of other malignancies, will be needed before it can be widely accepted as a chemoprevention strategy.
3. The best timing for PARP inhibitor use has yet to be defined with respect to the frontline, maintenance, or recurrent-disease setting. No formal data are available from the frontline setting yet. If we determine that PARP inhibition increases the likelihood of curing EOC, the frontline setting would be preferred. If, as seems more likely, PARP inhibition becomes another tool to prolong survival of EOC but does not cure it, then reserving use for the recurrent setting when minimizing toxicity is even more of a priority seems reasonable.
4. The best strategy for employing PARP inhibitors has yet to be defined with respect to monotherapy (intermittent or continuous), combination with chemotherapy, and/or combination wisth other targeted agents. In particular, appropriate dosing of both the PARP inhibitor and the other agent(s) in a combination approach needs to be determined.
5. The specific population that benefits from PARP inhibitor therapy has yet to be defined. The group whose cancer responds appears to be much broader than simply those with a germline BRCA1/2 mutation, the original population targeted. Expanding the study population to include high-grade serous tumors was the next consideration, with the goal of capturing the hereditary tumors in women who chose not to undergo genetic testing and the sporadic tumors thought to be most similar to BRCA-deficient ones. However, histology alone does not accurately predict BRCA mutation status. One large study found that only 45% of BRCA-mutated EOC had serous histology, although other studies suggest that 10% to 20% may be the more accurate estimate.[4,24,25] Notably, the NCCN guidelines recommend genetic counseling based on the diagnosis of EOC, FTC, or PPC, with no restriction on serous histology. The next line of thinking was that only platinum-sensitive cancers would respond to PARP inhibition. Although more data are needed regarding response to PARP inhibition in platinum-resistant EOC, platinum sensitivity alone does not seem to predict PARP inhibitor response. In the future, the ability to quickly and reliably classify tumors by the presence or absence of a homologous recombination defect (through either germline or somatic mutations) may predict subsequent response to PARP inhibition.
The issue of defining the population best served by PARP inhibition is only one example of a pressing need to prospectively allocate EOC patients to the most appropriate therapy. The Cancer Genome Atlas data have shown that, at least for serous EOC, no single driver mutation (or even small group of mutations) provides easily druggable targets for individualized therapy.[5] This differs from the situation in gastrointestinal stromal tumors, melanoma, and lung cancer, among other examples.
The need for predictive biomarkers only increases as we gain insight into the biology of EOC. Traditionally, all types of EOC, FTC, and PPC have been grouped together in clinical trials and treated similarly. Molecular studies now demonstrate, however, that there are distinct subtypes, including high-grade serous, low-grade serous, clear cell, and mucinous EOC. Debate is ongoing as to where endometrioid EOC tumors belong, since on the one hand, they cluster with high-grade serous tumors in many profiling studies, but on the other hand they often share a gene mutation (ARID1A) and a precursor lesion (endometriosis) with clear cell cancers.[26-29] As a clinical trial community, we will have to design studies that allow a cancer that is already relatively rare to be classified into even smaller groups based on histology and/or molecular pathways; otherwise, we run the risk of only identifying therapies that are effective in the dominant serous subtype and overlooking therapies that work in smaller subpopulations.[29]
6. Drug development presents challenges in the current health care economy, particularly when it is based on identifying a relatively small population that will benefit. Besides the well-known costs of initial research and development followed by the stepwise clinical trial process, multiple issues from the PARP inhibition story are informative about the overall dose-development environment.
The pathway to registration for a PARP inhibitor with the FDA is complex. Orphan drugs can be considered for registration based on phase II results, given the difficulty of mounting phase III trials in rare conditions and the frequent lack of alternative therapies. Although BRCA-deficient tumors are only a subset of EOC and breast cancer as a whole, they are likely too common to qualify for orphan drug eligibility. In addition, since there are other treatment options that offer potential benefit, a randomized phase III trial would likely be needed for registration.[30,31]
Based on the available phase I and II data, olaparib seems to be the PARP inhibitor most poised for phase III development. It currently is formulated as a 50-mg capsule. The recommended dose is 400 mg twice daily, however, meaning that a patient has to swallow 16 capsules a day. In order to simplify dosing, the manufacturer, AstraZeneca, is reformulating olaparib into higher-dose capsules. The reformulation process, however, may delay clinical trial development significantly.
In addition, if a PARP inhibitor's registration is dependent on identifying patients with germline BRCA mutations, the genetic test becomes a companion diagnostic test that requires FDA approval. Myriad Genetics, which holds the patent on commercial BRCA testing in the US, would have to pursue FDA approval distinct from its current Clinical Laboratory Improvements Amendments certification.[31]
REFERENCE GUIDE
Therapeutic Agents
Mentioned in This Article
AG014699/PF-01367338
BMN-673
Carboplatin
Cediranib (AZD2171)
CEP-9722
Gemcitabine
GP121016
Iniparib (BSI-201)
INO-1001
Irinotecan
MK4827
Olaparib (KU59436, AZD2281)
Paclitaxel
Pegylated liposomal doxorubicin
Temozolomide (Temodar)
Topotecan
Veliparib (ABT-888)
Brand names are listed in parentheses only if a drug is not available generically and is marketed as no more than two trademarked or registered products. More familiar alternative generic designations may also be included parenthetically.
A phase III trial with a PARP inhibitor in recurrent EOC would likely be the next step in development because the response in EOC has been more consistent than in breast cancer. The choice of drug for the standard-of-care arm then becomes relevant. Two randomized phase II trials have utilized PLD as the comparator to either olaparib or veliparib (NCT00628251, NCT01113957). Emerging data suggest that the results from these trials might be difficult to interpret due to the high response rate to PLD seen among BRCA mutation carriers. In a heavily pretreated population, Adams et al saw a 57% response rate to PLD among mutation carriers, compared with a rate of 20% among patients with sporadic EOC.[32] This response was associated with significantly improved progression-free and overall survival. Safra et al also documented marked differences in time to treatment failure and overall survival favoring mutation carriers after PLD treatment.[33] Notably, both groups found that PLD response appeared to be independent of platinum sensitivity. When combined with the well-documented improved sensitivity to platinum among mutation carriers, survival calculations in studies including this population must account for their propensity to survive longer, be exposed to additional chemotherapy regimens, and have longer treatment-free intervals than non–mutation carriers.[32] This underscores the need to have as many EOC, FTC, and PPC patients as possible appropriately classified with respect to germline (and possibly somatic) mutation status, not only so that their families can manage their cancer risk proactively, but also so that clinical trial planners can account for mutation carriers in the study design and interpretation.
Summary
PARP inhibitors have been shown to have activity in EOC, FTC, and PPC in the phase I and II setting. Responses have been seen in both BRCA-deficient and sporadic tumors and do not appear to require platinum sensitivity. Although PARP inhibitors have been well-tolerated as monotherapy, additional study is required to determine the efficacy and toxicity of PARP inhibitors in combination with chemotherapy and other targeted agents.
Without question, better understanding of the molecular events that underlie the development of EOC will improve outcomes. The ability to define a high-risk population has contributed significantly to this effort. PARP inhibition started as a rational drug development process to target germline BRCA-mutated malignancies, but has expanded to include tumors with somatic mutations in BRCA1/2 and, potentially, the entire homologous recombination pathway. The traditional concept of treating EOC as a single entity is giving way to recognition that clinical trials will have to address the remarkable heterogeneity in histology and molecular pathways that these tumors present. Unfortunately, the process of separating tumors by histology and altered pathways is not clear-cut, since tumors of different histology can have the same mutation and vice versa.
The incredible motivation of the community of EOC researchers, clinicians, and patients to find better answers for this disease has resulted in remarkably rapid accrual to the trials reviewed here. Although there are significant obstacles to overcome in the continued evaluation of PARP inhibition in EOC, FTC, and PPC, the unprecedented mobilization of the EOC community should bolster efforts to resolve those challenges.
Financial Disclosure: Dr. Zorn is principal investigator at UPMC for NCT01113957, which is sponsored by Abbott.
References:
References
1. Comen EA, Robson M. Inhibition of poly(ADP-ribose) polymerase as a therapeutic strategy for breast cancer. Oncology (Williston Park). 2010;24:1-10.
2. Rios J, Puhalla S. PARP inhibitors in breast cancer: BRCA and beyond. Oncology (Williston Park). 2011;25:1-11.
3. Dobzhansky T. Genetics of natural populations: recombination and variability of Drospohila pseudoobscura. Genetics. 1946;31:269-90.
4. Hennessy BT, Timms KM, Carey MS, et al. Somatic mutations in BRCA1 and BRCA2 could expand the number of patients that benefit from poly(ADP-ribose) polymerase inhibitors in ovarian cancer. J Clin Oncol. 2010;28;3570-6.
5. Cancer Genome Atlas Research Network. Integrated genomic analyses of ovarian carcinoma. Nature. 2011;474; 609-15.
6. Fong PC, Boss DS, Yap TA, et al. Inhibition of poly(ADP-ribose) polymerase in tumors from BRCA mutation carriers. N Engl J Med. 2009;361:123-34.
7. Fong PC, Yap TA, Boss DS, et al. Poly(ADP-ribose) polymerase inhibition: frequent durable responses in BRCA carrier ovarian cancer correlating with platinum-free survival. J Clin Oncol. 2010;28:2512-19.
8. Audeh MW, Carmichael J, Penson RT, et al. Oral poly(ADP-ribose) polymerase inhibitor olaparib in patients with BRCA1 or BRCA2 mutations and recurrent ovarian cancer: a proof-of-concept trial. Lancet. 2010;376:245-51.
9. Gelmon KA, Tischkowitz M, Mackay H, et al. Olaparib in patients with recurrent high-grade serous or poorly differentiated ovarian carcinoma or triple-negative breast cancer: a phase 2, multicentre, open-label, non-randomised study. Lancet. 2011;12:852-61.
10. Palma JP, Rodriguez LE, Bontcheva-Diaz VD, et al. The PARP inhibitor, ABT-888, potentiates temozolomide: correlation with drug levels and reduction in PARP activity in vivo. Anticancer Res. 2008;28:2625-35.
11. Palma JP, Wang, YC, Rodriguez LE, et al. ABT-888 confers broad in vivo activity in combination with temozolomide in diverse tumors. Clin Cancer Res. 2009;15:7277-90.
12. Schelman WR, Sandhu SK, Moreno Garcia V, et al. First-in-human trial of poly(ADP-ribose) polymerase (PARP) inhibitor MK-4827 in advanced ovarian cancer patients with antitumor activity in BRCA-deficient tumors and sporadic ovarian cancers (SOC). J Clin Oncol. 2011;29(suppl): abstract 3102.
13. Drew Y, Ledermann JA, Jones A, et al. Phase II trial of the poly(ADP-ribose) polymerase (PARP) inhibitor AG-014699 in BRCA 1 and 2 mutated, advanced ovarian and/or locally advanced or metastatic breast cancer. J Clin Oncol. 2011;29(suppl): abstract 3104.
14. Kaye S, Kaufman B, Lubinski J, et al. Phase II study of the oral PARP inhibitor olaparib (AZD2281) versus liposomal doxorubicin in ovarian cancer patients with BRCA1 and/or BRCA2 mutations. Presented at the 35th European Society for Medical Oncology (ESMO) Congress, Milan, Italy, October 8-12, 2010. Abstract 3725.
15. Ledermann JA, Harter P, Gourley C, et al. Phase II randomized placebo-controlled study of olaparib (AZD2281) in patients with platinum-sensitive relapsed serous ovarian cancer (PSR SOC). J Clin Oncol. 2011:29(suppl): abstract 5003.
16. Lee J, Squires J, Hays JL, et al. A pharmacokinetics/pharmacodynamics study of sequence specificity of the PARP inhibitor olaparib with carboplatin in refractory/recurrent women's cancers: NCT01237067. J Clin Oncol. 2011;29(suppl): abstract TPS158.
17. Hays JL, Kim G, Mariani J, et al. Sequence specific effects on DNA and cell damage with the PARP inhibitor olaparib (AZD2281) and carboplatin. J Clin Oncol. 2011;29(suppl): abstract 5025.
18. Lee J, Annunziata CM, Minasian LM, et al. Phase I study of the PARP inhibitor olaparib (O) in combination with carboplatin (C) in BRCA 1/2 mutation carriers with breast (Br) or ovarian (Ov) cancer (Ca). J Clin Oncol. 2011;29(suppl): abstract 2520.
19. LoRusso P, Ji JJ, Li J, et al. Phase I study of the safety, pharmacokinetics, and pharmacodynamics od the poly(ADP-ribose) polymerase inhibitor veliparib (ABT-888) in combination with irinotecan in patients with advanced solid tumors. J Clin Oncol. 2011:29(suppl): abstract 3000.
20. Penson RT, Whalen C, Lasonde B, et al. A phase II trial of iniparib (BSI-201) in combination with gemcitabine/carboplatin (GC) in patients with platinum-sensitive recurrent ovarian cancer. J Clin Oncol. 2011;29(suppl): abstract 5004.
21. Birrer MJ, Konstantinopoulos P, Penson RT, et al. A phase II trial of iniparib (BSI-201) in combination with gemcitabine/carboplatin (GC) in patients with platinum-resistant recurrent ovarian cancer. J Clin Oncol. 2011;29(suppl): abstract 5005.
22. Liu J, Fleming GF, Tolaney SM, et al. A phase I trial of the PARP inhibitor olaparib (AZD2281) in combination with the antiangiogenic cediranib (AZD2171) in recurrent ovarian or triple-negative breast cancer. J Clin Oncol. 2011;29(suppl): abstract 5028.
23. Ji J, Lee MP, Kadota M, et al. Pharmacodynamic and pathway analysis of three presumed inhibitors of poly(ADP-ribose) polymerase: ABT-888, AZD2281, and BSI201. Proceedings of the 102nd Annual Meeting of the American Association for Cancer Research. Orlando, FL, April 2-6, 2011. Abstract 4527.
24. Alsop K, Fereday S, Meldrum C, et al. Germline BRCA mutations in high-grade ovarian cancer: a case for routine BRCA mutation screening after a diagnosis of invasive ovarian cancer. J Clin Oncol. 2011;29(suppl): abstract 5026.
25. Lakhani SR, Manek S, Penault-Llorca F, et al. Pathology of ovarian cancers in BRCA1 and BRCA2 carriers. Clin Cancer Res. 2004;10:2473-81.
26. Gourley C, Michie CO, Keating KE, et al. Establishing a molecular taxonomy for epithelial ovarian cancer (EOC) from 363 formalin-fixed paraffin-embedded (FFPE) specimens. J Clin Oncol. 2011;
29(suppl): abstract 5000.
27. Wiegand KC, Shah SP, Al-Agha OM, et al. ARID1A mutations in endometriosis-associated ovarian carcinomas. N Engl J Med. 2010;363:1532-43.
28. Jones S, Wand TL, Shih leM, et al. Frequent mutations of chromatin remodeling gene ARID1A in ovarian clear cell carcinoma. Science. 2010;330:228-31.
29. Vaughan S, Coward JI, Bast Jr RC. Rethinking ovarian cancer: recommendations for improving outcomes. Nature Rev. 2011;11:719-25.
30. Balmana J, Domcheck SM, Tutt A, et al. Stumbling blocks on the path to personalized medicine in breast cancer: the case of PARP inhibitors for BRCA 1/2-associated cancers. J Cancer Discov. 2011;1:29-34.
31. Domchek SM, Mitchell G, Lindeman GJ, et al. Challenges to the development of new agents for molecularly defined patient subsets: lessons from BRCA 1/2-associated breast cancer. J Clin Oncol. 2011;29:4224-6.
32. Adams SF, Marsh EB, Elmasri W, et al. A high response rate to liposomal doxorubicin is seen among women with BRCA mutations treated for recurrent epithelial ovarian cancer. Gynecol Oncol. 2011;123:486-91.
33. Safra T, Borgato L, Nicoletto MO, et al. BRCA mutation status and determinant of outcome in women with recurrent epithelial ovarian cancer treated with pegylated liposomal doxorubicin. Mol Cancer Ther. 2011;10:2000-7.