Pharmaceutical mergers raise fears of Rx pipeline pruning
The likely consolidation of four international drug companies has raised concerns about the range of oncology agents that will remain in the R&D pipeline once the newly merged companies streamline their operations.
ABSTRACT: The planned mergers of Merck with Schering-Plough and Pfizer with Wyeth seem to be about plugging pipeline gaps, not growing an internal R&D engine, experts say.
The likely consolidation of four international drug companies has raised concerns about the range of oncology agents that will remain in the R&D pipeline once the newly merged companies streamline their operations.
Workforce consolidation may bring short-term cost savings, but if the R&D labor force is reduced, then efforts may be focused on fewer drugs but emphasize blockbusters, said Robert Catalano, PharmD, regulatory officer with the Eastern Cooperative Oncology Group in Philadelphia, one of the largest cancer clinical research organizations in the U.S. A blockbuster mentality could harm oncology, where one-drug-treats-all strategies are being replaced by targeted treatments.
“Take breast cancer, for example. We now have at least five molecular subtypes of breast cancer, and that limits the type of drugs that will be used,” Dr. Catalano said. “Companies may shy away from looking at those agents that have a smaller market share potential.”
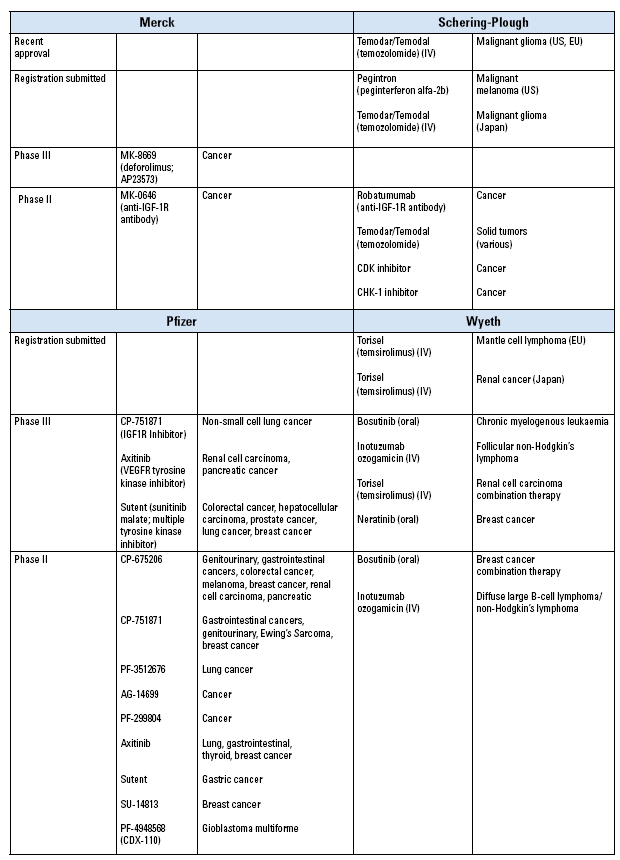
Merck announced that it will buy Schering-Plough for $41.1 billion, a deal that Merck expects to result in cost savings of approximately $3.5 billion per year beyond 2011.
Meanwhile, Pfizer will acquire rival Wyeth in a $68 billion buyout. Despite assurances that the aim is to create a “broad, diversified portfolio,” Pfizer has stated that it wants to make $4 billion in “synergies” by 2012, shrinking the combined workforce of the two companies by 15%.
The planned mergers seem to be about plugging pipeline gaps not growing an internal R&D engine, said Keith Blundy, PhD, CEO of London-based Cancer Research Technology. “I suspect that they will look to save costs by cutting out duplication. Even though they may have been addressing the same target, different agents will act differently. Clearly, they are not going to run all options,” he said.
As of March 2009, the companies declined to discuss with Oncology New International how the planned mergers will specifically affect their oncology R&D strategies or which, if any, cancer drugs will be weeded out from the development process. Until the acquisitions are completed, a process that is likely to go into the fourth quarter 2009, all four will continue operating as separate entities.
Initial signs of belt-tightening have nonetheless been observed. Pfizer has announced closure of five of its 46 manufacturing plants. A hiring freeze has been instituted at Merck and Schering-Plough.
Instances of duplication in the oncology pipeline are being identified as well. Both Merck and Schering-Plough have an IGF-1R monoclonal antibody in phase II trials: MK-0646 (Merck) and robatumumab (Schering-Plough). It remains to be seen how long this tandem development will continue.
“(This is) one of the few examples of where the two companies have a compound with the same mechanism of action in clinical development. In this case, this will allow us to select the compound with the best characteristics,” said Peter Kim, PhD, president of Merck Research Laboratories.
Pfizer insists that Wyeth’s “robust research program” will be maintained after the takeover. “Pfizer worldwide research will continue to focus and prioritize its resources and investments in high-growth areas including oncology,” according to Martin Fensch, head of corporate media relations for Pfizer Oncology Europe.
Wyeth has just moved its dual HER-2 and EGFR kinase inhibitor, neratinib, into phase III after reporting positive phase II results at SABCS 2008 (abstract 37).
In another positive development, Pfizer has halted its phase III trial of Sutent (sunitinib malate) in patients with advanced pancreatic islet cell tumors after the drug showed significant benefit in this rare cancer.
Sutent is currently approved for advanced renal cell carcinoma and second-line gastrointestinal stromal tumors. Phase III trials of additional indications are ongoing.
If the Merck/Schering-Plough deal goes ahead as planned, Merck will benefit from the February 2009 approvals by the European Commission and the FDA for an IV formulation of Schering-Plough’s temozolomide. The chemotherapy agent, which is marketed as Temodar in the U.S. and Temodal in the EU, is already approved in oral capsule form for the treatment of malignant glioma.
Merck is also waiting on a supplemental biologics license that would allow its own cervical cancer vaccine, Gardasil, to be used in boys and young men for prevention of external genital lesions caused by human papillomavirus. The application was submitted to the FDA in December 2008.
Steps to broaden the indications for Schering-Plough’s peginterferon alfa-2b (Cylatron/Pegintron) appear to have stalled, though. Schering-Plough has withdrawn its EU marketing authorization application for the drug to be used as adjuvant treatment for patients with stage III melanoma.
The application was initially submitted to the European Medicines Agency back in September 2007. FDA approval has not yet been granted either.
“Schering-Plough received a Complete Response Letter from the FDA regarding its supplemental biologics license application in late March 2008. Schering-Plough is working closely with the FDA to address their request and intends to submit its response as soon as possible. A new review period will begin once the additional information is submitted to the FDA,” a company spokesperson said.
On April 3, Pfizer announced that it received a request from the U.S. Federal Trade Commission (FTC) for additional information with respect to the Wyeth acquisition.
Meanwhile, Wyeth and the National Cancer Coalition have launched a pilot program to assist cancer patients in Latin America with donations of Mylotarg (gemtuzumab ozogamicin for injection) and Neumega (oprelvekin).
How Supportive Care Methods Can Improve Oncology Outcomes
Experts discussed supportive care and why it should be integrated into standard oncology care.