Product Profile of Amivantamab Plus Chemotherapy in EGFR Exon 20+ NSCLC
Tammy McClellan, PharmD, discusses the use of amivantamab plus chemotherapy in patients with non–small cell lung cancer harboring EGFR exon 20 insertion mutations.
Tammy McClellan, PharmD
Clinical Oncology Pharmacist, Riverside Healthcare, Kankakee, IL

ONCOLOGY spoke with Tammy McClellan, PharmD, about the recent approval of amivantamab-vmjw (Rybrevant) plus chemotherapy. She focused on adverse effects patients may experience from the combination and how administration and dosing may play a role in any challenges observed.
The product profile of amivantamab plus chemotherapy in patients with
non–small cell lung cancer harboring EGFR exon 20 insertion mutations.
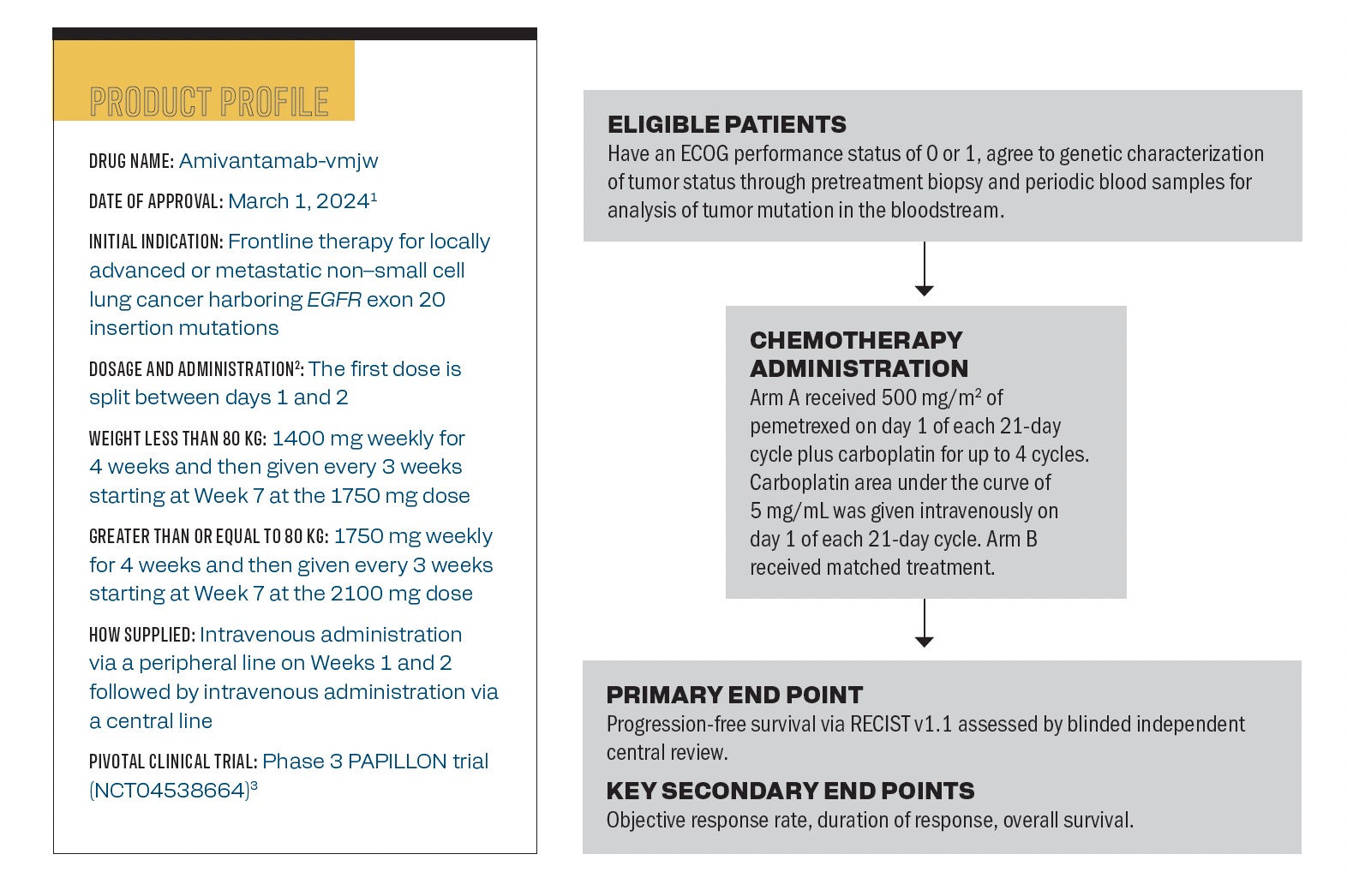
Q / How is amivantamab dosed and administered alongside chemotherapy in the first-line setting?
McClellan / This is a weight-based drug, and the cutoff is 80 kg. To be more specific, if you’re less than 80 kg, or if you’re greater than or equal to 80 kg. [It’s administered as] cycle 1 days 1, 2, 8, and 15. During cycle 1, day 1 all patients receive 350 mg of amivantamab.[For patients who weigh] less than 80 kg, 1050 mg on day 2 followed by 1400 mg weekly for 3 weeks during cycle 1, days 8 and 15, and cycle 2 day 1. Then it is given every 3 weeks starting at Week 7 which is the same as cycle 3 Day 1 at the 1750 mg dose, with no doses during weeks 5 and 6. For patients who weigh greater than or equal to 80 kg, 1400 mg is given on day 2 followed by 1750 mg weekly for 3 weeks during cycle 1, days 8 and 15, and cycle 2, day 1. It is then given every 3 weeks starting at week 7 which is the same dose as cycle 3 day 1 at the 2100 mg dose, with no doses on weeks 5 and 6.
Q / Are there any adverse effects or toxicities that clinicians should be aware of with amivantamab?
McClellan / There is a higher incidence of infusion-related reactions. That is the reason why you would split your initial dose between days 1 and 2. It is also recommended that you do not use a central line for the first 3 doses- a peripheral line is used instead. This is a conscious effort to reduce the severity of infusion-related reactions. Other reactions include interstitial lung disease/pneumonitis, ocular toxicities (keratitis, dry eye symptoms, conjunctival redness, blurred vision, visual impairment, ocular itching, uveitis)-which seem to be more and more common with the newer immunotherapy agents, dermatologic adverse reactions like dermatitis, pruritus, and dry skin. This drug may cause photosensitivity so patients would want to avoid/minimize exposure. This drug can cause nail toxicity, neurologic disorders, adverse gastrointestinal effects, peripheral neuropathy, mucositis, edema, fatigue, weight loss, and musculoskeletal pain. Amivantamab does have an adverse reaction of hemorrhoids. We hear about constipation, but not necessarily hemorrhoids and I wanted to point that out.
Q / What laboratory monitoring and treatment strategies are recommended for patients receiving amivantamab plus chemotherapy?
McClellan / The standard complete blood count is needed to account for the different types of myelosuppression and/or infection. The comprehensive metabolic panel is needed to assess the various electrolyte imbalances, serum glucose abnormalities, hepatotoxicity, nephrotoxicity, albumin, magnesium and gamma-glutamyl transferase (GGT) levels. GGT levels can be controversial but I would like to mention that soHealthcare Providers can make their own decisions in light of the reported data.
Q / Are there any significant drug interactions between amivantamab and commonly used medications in patients with cancer?
McClellan / In the package insert,2 there are no drug interactions listed. However, it is given in combination with pemetrexed [and carboplatin], which do have known interactions with nonsteroidal anti-inflammatory drugs. When given in combination [with another drug], I would look at the possibilities of synergistic adverse effects by assessing the administration of concurrent hepatotoxic and nephrotoxic agents. However, [because] it does have some parallel toxic effects, I would monitor any other concurrent hepatotoxic drugs that may be administered, just for that synergistic effect, if you will.
Q / Because this treatment was recently approved, have there been any access or logistical challenges associated with administering amivantamab plus chemotherapy?
McClellan / I would like to mention the infusion times and likelihood of the heightened incidence of the infusion reactions. The precautions that we are aware of include initial split dosing, several administration rates, and long infusion times until you reach the plateau-you have to take your time with this. With that, just be diligent and watch your patient. You most definitely should have a 1:1 nurse-to-patient ratio, if not a 2:1, so one [nurse can administer] while the other one is helping out [during the infusion], to at least get through the first administration on days 1 or2.
[This treatment is given in the outpatient setting], however, [it is important to have] everything in place, making sure the premedications are given within the recommended times: diphenhydramine at 25 mg to 50 mg or equivalent and acetaminophen at 650 mg to 1000 mg- prior to each infusion, dexamethasone at 20 mg or equivalent- for the first infusion followed by dexamethasone 10 mg or equivalent for the second dose and thereafter optional use per the manufacturer.
Consideration of an H2 blocker to provide a different mechanism of action to combat infusion reactions is warranted. We express to the patient the importance of if you feel anything report it.
Q/ How might the role of amivantamab or similar targeted therapies evolve in the treatment landscape for EGFR-mutated NSCLC?
McClellan / I am biased when it comes to non–small cell lung cancer as I would describe this disease state as one of the best examples when it comes to implementing precision medicine, targeted therapy, and genetic testing through the use of identifying relevant biomarkers and all that oncology health care providers are trying to accomplish. Fighting this cancer by treating it specifically, limiting the adverse effects, and giving the patient the best chance at obtaining a cure or an improvement in their quality of life. It excites me to have a great collaboration of precision medicine and targeted therapy for the individual all while providing tailored health care.
References
- FDA approves amivantamab-vmjw for EGFR exon 20 insertion-mutated non-small cell lung cancer indications. News release. FDA. March 1, 2024. Accessed March 1, 2024. https://tinyurl.com/msw4u5yk
- Rybrevant. Prescribing information. Janssen; 2021. Accessed May 13, 2024. https://shorturl.at/kRWZ0
- Zhou C, Tang KJ, Cho BC, et al; PAPILLON Investigators. Amivantamab plus chemotherapy in NSCLC with EGFR exon 20 insertions. N Engl J Med. 2023;389(22):2039-2051. doi:10.1056/NEJMoa2306441
