Promising Antitumor Activity Observed Following Treatment With Eftilagimod Alpha Plus Pembrolizumab for ICI-Resistant Advanced NSCLC
A challenging patient population with advanced non–small cell lung cancer and resistance to common immunotherapeutics appeared to derive promising benefit from treatment with eftilagimod alpha plus pembrolizumab.
Second-line treatment with the soluble LAG-3 protein eftilagimod alpha in combination with pembrolizumab (Keytruda) yielded promising antitumor responses in a population of patients with metastatic non–small cell lung cancer with limited treatment options following progression on a PD-1/L1 inhibitor, according to findings from the phase 2 TACTI-002 trial (NCT03625323) presented at the 2022 World Conference on Lung Cancer.
The treatment yielded an overall response rate of 5.6% in the intent-to-treatment population (n = 36) by both iRECIST and RECIST 1.1 criteria, all of which were partial responses. A total of 11 and 10 patients, respectively, achieved stable disease by each criterion and 22 and 23 patients had progressive disease. Moreover, the disease control rate was 36.1% by iRECIST criteria and 33.3% by RECIST 1.1 criteria.
Eftilagimod alpha targets a subset of MHC class II molecules that mediate activation of antigen presenting, natural killer, and T cells. Investigators believed that stimulating the dendritic cell network that leads to T-cell recruitment and activation could be a viable means to overcome PD-1 inhibitor resistance.
Eftilagimod alpha was administered at a dose of 30 mg subcutaneously every 2 weeks for the first 8 three-week cycles followed by once per cycle for the next 9 cycles plus pembrolizumab at a 200-mg dose every 3 weeks for 2 years. A total of 36 patients were included in the study from April 2019 to August 2021.
Investigators reported that 75% of patients had PD-L1–low, defined as a tumor proportion score of 1% to 49%, or PD-L1–negative disease. Moreover, 66.7% of patients were previously treated with a regimen consisting of chemotherapy plus an anti–PD-1 or PD-L1, with 41.7% not responding to their first line treatment. The median patient age was 67 years (range, 46-84) and most patients were men (61.1%). Additionally, most patients were current or previous smokers (86.1%) with nonsquamous pathology (77.8%).
The median overall survival (OS) in the intent to treat population was 9.7 months with a OS rate of 72.2% at 6 months,
TABLE. A challenging patient population with advanced non–small cell lung cancer and resistance to common immunotherapeutics appeared to derive promising benefit from treatment with eftilagimod alpha plus pembrolizumab.
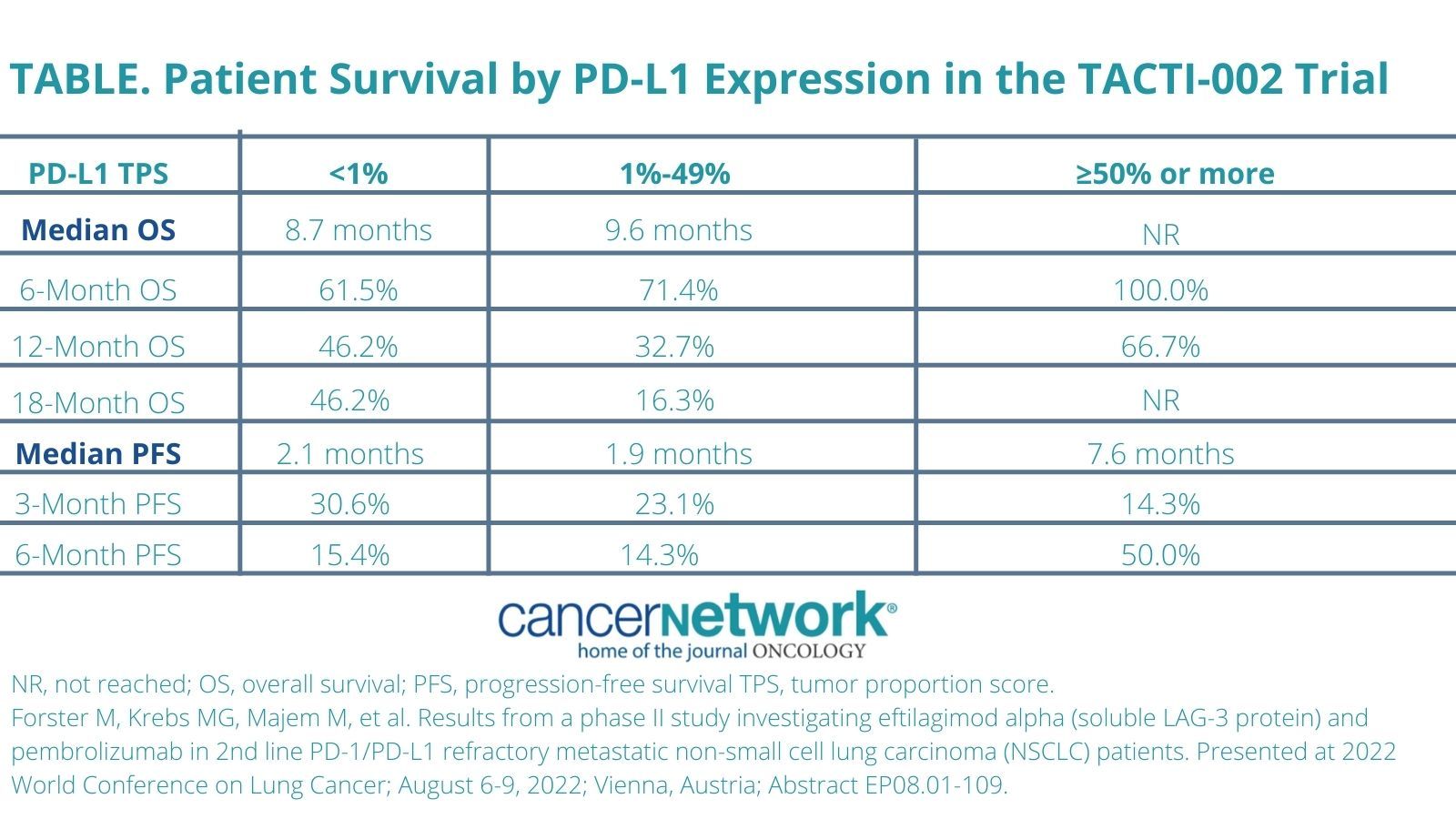
43.4% at 12 months, and 36.5% at 18 months. Moreover, investigators also reported outcomes by PD-L1 expression. OS results were also reported based on PD-L1 expression (Table).
Adverse effects (AEs) of any grade were reported in 97.2% of patients, with 19.4% experiencing any serious AEs. Grade 3 or higher AEs occurred in 33.3% of patients. Two fatalities occurred (5.6%), neither of which were related to treatment.
Common toxicities of any grade included decreased appetite (33.3%), dyspnea (30.6%), cough (27.8%), asthenia (22.2%), and fatigue (19.4%). Grade 3 AEs included dyspnea (2.8%), asthenia (2.8%), fatigue (2.8%), and arthralgia (2.8%), with 1 event of grade 4/5 dyspnea (2.8%). Any-grade AEs associated with treatment included asthenia (13.9%), injection site erythema (13.9%), and injection site reaction (11.1%). There were no treatment-related death or drug discontinuations.
Reference
Forster M, Krebs MG, Majem M, et al. Results from a phase II study investigating eftilagimod alpha (soluble LAG-3 protein) and pembrolizumab in 2nd line PD-1/PD-L1 refractory metastatic non-small cell lung carcinoma (NSCLC) patients. Presented at: 2022 World Conference on Lung Cancer; August 6-9, 2022; Vienna, Austria; Abstract EP08.01-109.