Recap: CLEAR Trial Regimen as a Standard for Frontline Clear Cell RCC: Impressive Efficacy but Personalization Is Needed
A panel of experts discuss the updated results of the phase 3 CLEAR trial presented at the 2022 International Kidney Cancer Symposium.

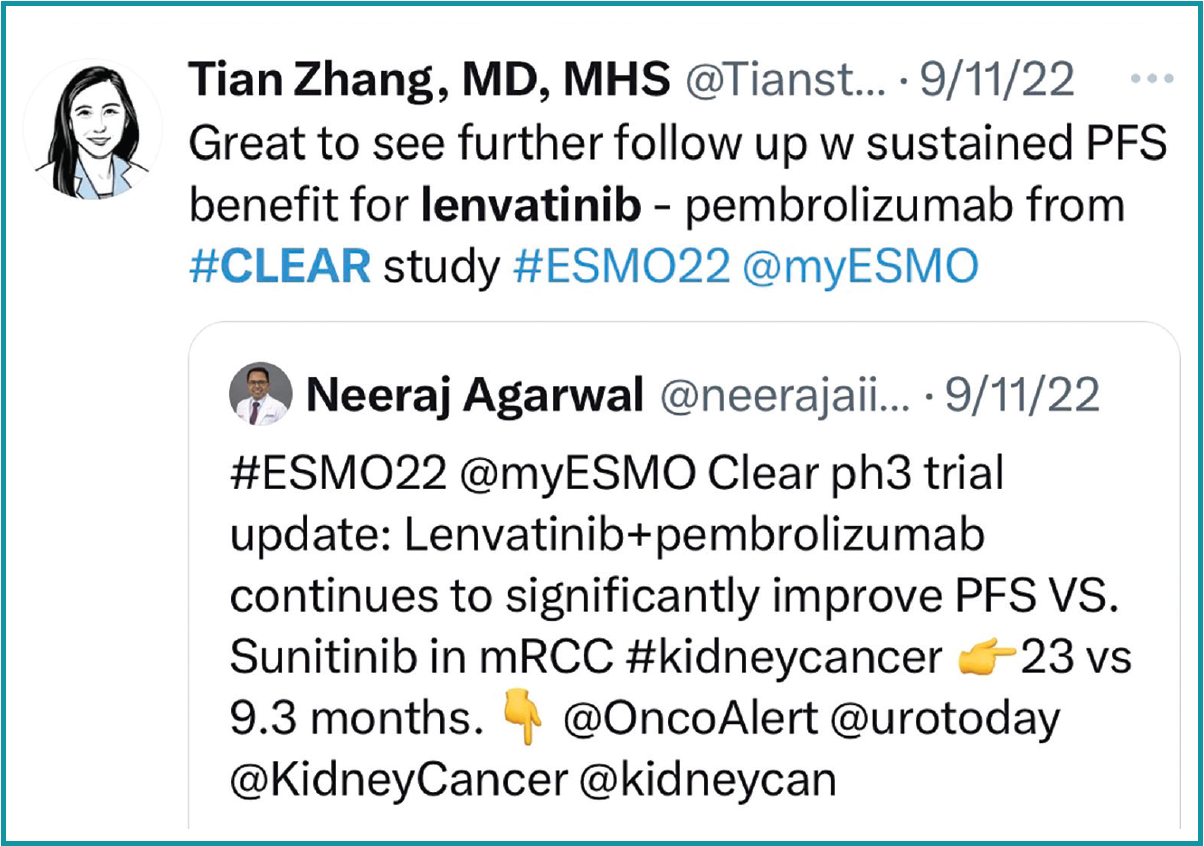
When discussing treatment options for frontline therapy in clear cell renal cell carcinoma (RCC), the panel of experts turned to results of the phase 3 CLEAR trial (NCT02811861) analyzing lenvatinib (Lenvima) plus pembrolizumab (Keytruda) or everolimus (Afinitor) vs sunitinib (Sutent) in the aforementioned patient population.1
Specifically, they focused on the updated study results presented at the 2022 International Kidney Cancer Symposium. Patients were randomly assigned 1:1:1 to receive 20 mg of oral lenvatinib daily plus 200 mg of intravenous pembrolizumab every 3 weeks, 18 mg of oral lenvatinib every day plus 5 mg of oral everolimus every day, or 50 mg of oral sunitinib every day for a 4-weeks-on, 2- weeks-off schedule.
The updated results presented at the conference showed a median progression-free survival (PFS) of 23.3 months (95% CI, 20.8-27.7) in the lenvatinib/ pembrolizumab arm vs 9.2 months (95% CI, 6.0-11.0) in the sunitinib arm (HR, 0.42; 95% CI, 0.34-0.52). The median overall survival (OS) was not reached in either arm (HR, 0.72; 95% CI, 0.55-0.93). Additionally, the 36-month OS rate was 94.5% for those who completed treatment with pembrolizumab.
The objective response rate was 71.0% (95% CI, 66.3%-75.7%) in the combination arm vs 36.1% (95% CI, 31.2%- 41.1%) in the sunitinib arm (response rate [RR], 1.97; 95% CI, 1.69-2.29). The median duration of response was 26.0 months (95% CI, 22.2-41.4) in the combination arm vs 14.7 months (95% CI, 9.4-16.8) in the sunitinib arm.
Additional findings from the analysis showed that patients who completed treatment with pembrolizumab continued being treated with lenvatinib, leading to continued clinical benefit.
ORNSTEIN: My initial thoughts when the CLEAR data first went out was that they were extremely impressive, both in regard to PFS, the HR for OS, the response rate, and the CR [complete response] rate as well. In fact, with those initial numbers, I don’t think many expected lenvatinib plus pembrolizumab as a standard within frontline RCC.
It’s always reassuring to see the updated data. I don’t think for me anything changed other than being comforted to see that the data are holding true even with additional follow-up. I didn’t see anything jump out that would sway me 1 way or the other either to use it more or to use it less based on the updated data.
My general impressions are that when you have a PFS of close to 2 years and an HR for OS that’s leveling out where the other regimens have and you see these response rates that exceed 70%, it’s certainly a reasonable regimen and one that I use fairly frequently in the frontline setting.
ZHANG: Lenvatinib and pembrolizumab certainly are standard for our patients in the frontline setting these days. I would note that all the frontline immunotherapy combination trials have been measured up against sunitinib, so none of them have been compared with each other. We use these depending on the patient in front of us. I will reach for lenvatinib and pembrolizumab for patients who need early disease control and can’t afford any disease progression on frontline therapy.
RINI: These data show an impressive 2-year PFS. For someone who strictly uses immunotherapy, would you give this regimen a chance?
HAMMERS: I don’t use [immunoimmunotherapy] in everyone. For patients who I feel have a larger disease burden where I want to have a response, this is my preferred regimen. I think it’s the most efficacious one if you were to compare it across clinical trials.
A higher response rate doesn’t necessarily mean that those patients are cured. If you look at the duration of response of 26 months if you treat somebody with pure immunotherapy, even after 5 years the median duration of response has not yet been reached.
There’s a difference obviously in the quality of response, but I love this regimen. The other thing that we haven’t mentioned—it’s a little bit odd in the efficacy data—is that [lenvatinib] or this particular [tyrosine kinase inhibitor] is uniquely combinable. We don’t see an increase in liver toxicity. I think that makes this personally my favorite regimen if I must go in that direction.
RINI: The 2-year PFS rate isn’t translating into even better survival vs other regimens, correct?
HAMMERS: The HR is 1 measure with how to statistically describe 2 curves, if you will. It’s not necessarily the perfect one, so I do think there is something to a median OS that they used to look at for decades. Then sometimes curves may overlap for a little bit before they separate, and that’s not the statistically best scenario to use as an HR. It’s 1 of the measures.
I think we need to still look at the whole curves and we also need to look at landmark analyses, time points, and late time points and see if there might be some difference to get the full impression of these OS benefits.
REFERENCE
- Potra CG. Updated efficacy of lenvatinib + pembrolizumab versus sunitinib in patients with advanced renal cell carcinoma (aRCC) in the CLEAR study. Presented at: 2022 IKCS: North America; November 4-5, 2022; Austin, Texas. Abstract 31.
