Reducing Skeletal-Related Events in Metastatic Castration-Resistant Prostate Cancer
Here we discuss the efficacy and safety of zoledronic acid, denosumab, enzalutamide, abiraterone, and radium-223 and review the available data regarding the cost of denosumab compared with that of zoledronic acid.
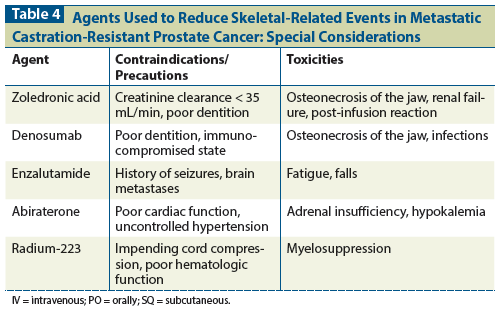
Table 1: Overview of Study Design in Studies of Agents That Showed Reduced Skeletal-Related Events in Men With Metastatic Castration-Resistant Prostate Cancer
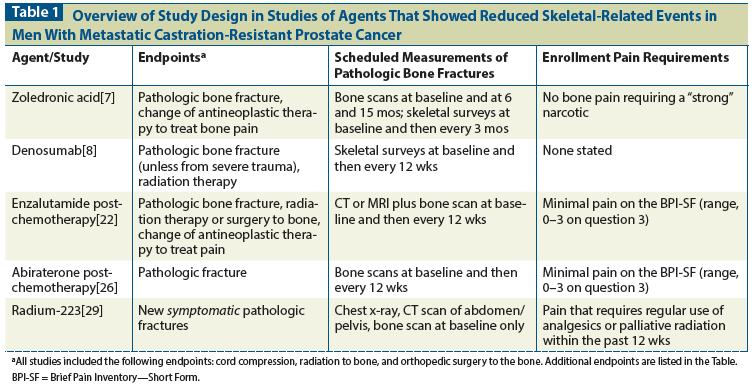
Table 2: Hazard Ratios for Time to First Skeletal-Related Event (SRE) or Symptomatic Skeletal Event (SSE) in Studies of Agents Used to Reduce SREs in Metastatic Castration-Resistant Prostate Cancer
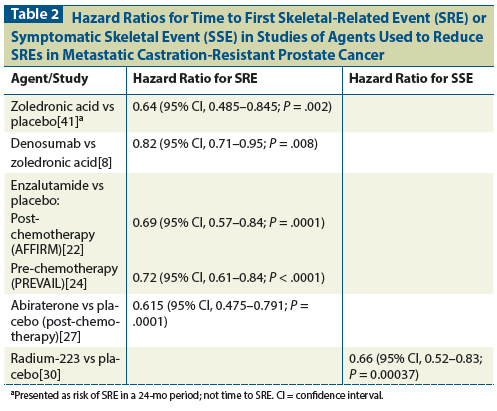
Table 3: Dose, Schedule, and Duration of Treatment for Agents Used to Reduce Skeletal-Related Events in Metastatic Castration-Resistant Prostate Cancer
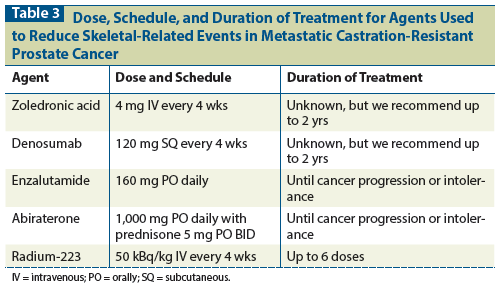
Table 4: Agents Used to Reduce Skeletal-Related Events in Metastatic Castration-Resistant Prostate Cancer: Special Considerations
Skeletal-related events contribute substantially to morbidity, mortality and cost in men with metastatic castration-resistant prostate cancer (mCRPC). There are five agents available for treatment in mCRPC that reduce skeletal-related events. Here we discuss the efficacy and safety of zoledronic acid, denosumab, enzalutamide, abiraterone, and radium-223. We include data on and a discussion of duration of treatment with zoledronic acid and denosumab, the only two of these agents that do not have a clinically proven anticancer effect. Finally, we review the available data regarding the cost of denosumab compared with that of zoledronic acid.
Epidemiology
In 2015, an estimated 220,800 US men will be diagnosed with prostate cancer and 27,540 will die of the disease, making prostate cancer the most common nondermatologic cancer in men and the second most deadly.[1] Among men who develop metastatic prostate cancer, up to 90% will have osseous metastases.[2]
Definitions of SRE and SSE
Osseous metastatic disease is common in metastatic prostate cancer and typically occurs in the axial skeleton and/or proximal appendicular skeleton, possibly because these bones contain hematologically active bone marrow.[3] Prostate cancer osseous metastases stimulate osteoblasts, resulting in blastic osseous metastases and elevated levels of serum alkaline phosphatase.[4] Events that occur because of osseous metastases are termed “skeletal-related events” (SREs) and include pathologic bone fractures, spinal cord compression, orthopedic surgery interventions, and palliative radiation to the bone. Some studies also include in the SRE definition change in neoplastic therapy secondary to bone pain. SREs may be detected clinically on the basis of symptoms or by reviewing imaging; detection via imaging is possible whether or not an SRE causes symptoms. Symptomatic skeletal events (SSE) use a slightly different definition that limits these to events that affect the patient experience. This definition was first used in the pivotal Alpharadin in Symptomatic Prostate Cancer (ALSYMPCA) trial, where SSEs included external beam radiation therapy to relieve bone pain, occurrence of new symptomatic pathologic fractures (vertebral and nonvertebral), occurrence of spinal cord compression, or tumor-related orthopedic interventions. Even when the SRE definitions are similar, their measurement may differ: eg, some studies have included routing skeletal radiography to detect asymptomatic events, while others have not.
Clinical Significance of SREs
SREs are associated with increased mortality and cost. In an examination of 194 patients with prostate cancer treated with androgen suppression therapy, 24 experienced a fracture after their diagnosis. Overall survival was significantly decreased in these patients compared with survival in those without fracture: median overall survival was 121 months in men with a fracture and 160 months in those without (P = .04).[5] A recent publication examined inpatient billings (from the Nationwide Inpatient Sample [NIS]) for SREs between 1998 and 2010 and found that the inpatient cost of SRE had increased by 94% over that period-to $369,256,799.[6] However, the rate of SRE decreased from 18% to 15.4%, and SRE-associated mortality also decreased, from 8.5% to 4.7%. The authors hypothesize that the markedly increased cost of treating SREs (relative to the rate of SRE) is related to inflated costs of the treatments for SRE, as well as the costs of treating comorbid conditions in affected patients.
Ways of Assessing SREs
Pathologic fracture
Detection of pathologic fracture-and the time to detection-in a clinical trial varies depending on the imaging modality used and on the frequency of imaging, respectively. Different types of imaging studies have different sensitivities for fracture, and the particular study used has varied from trial to trial. In some studies, prostate cancer patients received scheduled skeletal surveys that included plain films of the skull, spine, chest, pelvis, arm from shoulder to elbow, and leg from hip to knee.[7,8] The sensitivity of plain films for fracture varies depending on location, and they are likely to miss occult fractures.[9] Skeletal surveys are not commonly used in the management of prostate cancer, so their real-world applicability is limited. In most studies, nuclear medicine bone scans with technetium-99 were used. The sensitivity of a nuclear medicine bone scan for fractures is high; for example, such a scan has a sensitivity of 98% for an occult hip fracture.[9] However, bone scans also show blastic osseous metastatic disease, which could hide a fracture, given that fractures often occur in bones weakened by tumors. In studies that only required bone scans, fractures were likely detected by other means, such as CT scan or plain films that were ordered to follow up on pain. The frequency of imaging studies affects time to detection of pathologic fracture. Most of the studies included visits with patients every 4 weeks and imaging every 12 weeks.
Spinal cord compression
The most common presenting symptom for spinal cord compression is pain followed by neurologic changes.[10] Diagnosis often requires an MRI or CT myelogram.
Orthopedic surgery to the bone
This is detected by a review of patient records.
Palliative radiation to the bone
This is also detected by a review of patient records. Subjectivity is involved in the determination of this SRE, since pain in the bone may lead one clinician to refer a patient to radiation oncology, while another clinician might respond to the same complaint by changing the antineoplastic regimen.
Change of antineoplastic therapy for bone pain
This is also detected by a review of patient records. In addition, with this SRE, the investigator is required to document the reason that a change in therapy was made.
Agents That Reduce SREs and SSEs
Zoledronic acid
Zoledronic acid is an intravenous (IV) bisphosphonate administered over at least 15 minutes.[11] Dose reductions are required for reduced kidney function, and it is contraindicated in patients with a glomerular filtration rate (GFR) < 35 mL/min. Since many patients with metastatic castration-resistant prostate cancer (mCRPC) are elderly, and because the serum creatinine level can be misleading in older patients, the GFR should be calculated using the Cockcroft-Gault equation,[12] which takes into account age, gender, and weight.
A phase III randomized controlled study that enrolled patients from June 1998 to January 2001 examined zoledronic acid at a dose of 4 mg, zoledronic acid at a dose of 8 mg, or placebo, administered IV every 3 weeks for 20 cycles.[7] To participate, men were required to have bone metastases and evidence of castration-resistant disease, as evidenced by three consecutive increases in prostate-specific antigen (PSA) level while receiving androgen suppression therapy. Patients could not be receiving cytotoxic chemotherapy at the time of study entry, but it could be started during the study. Additionally, participants could not have bone pain requiring “strong” narcotic therapy; could not have received radiation therapy within 3 months or prior bisphosphonate therapy; and could not have significant kidney dysfunction (creatinine level > 3.0 mg/dL), abnormal calcium level, or refractory hypertension.
The primary outcome was the proportion of patients in each group who had at least one SRE (the outcomes described above, including change of antineoplastic regimen for bone pain). Bone scans were done at baseline and at months 6 and 15. Skeletal surveys (plain films) were done every 3 months. This study included central interpretation of imaging studies by radiologists blinded to treatment assignment. Secondary outcomes included time to SRE, proportions of patients with each individual type of SRE, skeletal morbidity, time to disease progression, objective bone lesion response, bone biochemical markers, and quality of life.
A total of 643 patients were enrolled and subsequently randomized to zoledronic acid, 4 mg (n = 214); zoledronic acid, 8 mg (n = 221); or placebo (n = 208). In June 2000, those randomized to the 8-mg group had their dose decreased to 4 mg because of excessive renal toxicity at the higher dose. There were 92 SREs (44.2%) in the placebo group and 71 (33.2%) in those in the zoledronic acid 4-mg group. The result in the group that started at 8 mg and later was switched to 4 mg (the “8/4 group”) was intermediate to these two and was subsequently de-emphasized in many reports. The zoledronic acid 4-mg arm had an 11% reduction in SREs, which was statistically significant (P = .021). Among those patients initially assigned to the 8-mg dose cohort, 85 (38.5%) had at least one SRE. The percentage of SREs in the 8/4-mg cohort was not significantly improved over that in the placebo cohort. Median time to SRE was 321 days in the placebo group, was not reached in the zoledronic acid 4-mg group, and was 363 days in the zoledronic acid 8/4-mg group. Only the difference between the zoledronic acid 4-mg and placebo groups was statistically significant (P = .011). There were no significant differences in quality of life scores or radiographic response.
There was more renal failure in patients receiving zoledronic acid than in the placebo group, particularly in the group that initially received 8 mg: 15.4% at the 4-mg dose, 20.7% at the 8/4-mg dose, and 11.5% with placebo. Eight patients treated with zoledronic acid experienced grade 3 or 4 hypocalcemia (four in each group). Other toxicities that occurred more frequently in the zoledronic acid groups included fatigue, anemia, myalgia, fever, and lower extremity edema. The rates of serious adverse events that led to treatment discontinuations were similar among the three groups: 9.8% at the 4-mg dose, 12.4% at the 8/4-mg dose, and 10.1% with placebo.
Osteonecrosis of the jaw (ONJ) was not reported in this study, likely because it was not a widely recognized entity at the time the study was published. ONJ typically presents as a nonhealing extraction socket or exposed jawbone and was described in conjunction with bisphosphonate therapy in a publication by a group of maxillofacial surgeons in 2004.[13] This group presented a series of 63 patients who presented with ONJ to the Long Island Jewish Medical Center and the University of Maryland from February 2001 to June 2003. Prior to 2001, these surgeons typically treated 1 or 2 patients with ONJ per year. Among the 63 patients treated in the indicated time period, 7 had osteoporosis without malignancy, and 56 had cancer. All of these patients had received a bisphosphonate. Fifty-four of the patients had had a dental procedure, but 9 developed spontaneous ONJ. The study described above was published prior to this description of ONJ and thus did not include ONJ as a toxicity. Estimates of ONJ in prostate cancer are hard to find and may be flawed,[14] but one longitudinal study of 41 prostate cancer patients receiving bisphosphonate therapy showed that 2 had ONJ (4.9%).[15]
The study of zoledronic acid is the oldest of the five trials and therefore the furthest removed from current antineoplastic therapy standards. Indeed, patients enrolled in this trial were not routinely treated with docetaxel, as it had not been approved for prostate cancer at that time.
Denosumab
Denosumab is a monoclonal antibody directed to receptor activator of nuclear factor kappa-B ligand (RANKL) that is given every 4 weeks at a dose of 120 mg subcutaneously (SQ) for mCRPC with bone metastases.[16] From 2006 to 2009, 1,904 subjects with mCRPC participated in an international clinical trial comparing zoledronic acid against denosumab.[8] Those in the zoledronic acid arm received 4 mg of zoledronic acid every 4 weeks plus a placebo SQ injection, and those in the denosumab arm received 120 mg of denosumab SQ plus a placebo IV infusion. Only patients with at least one bone metastasis, normal calcium, and good renal function (creatinine clearance > 30 mL/min) could participate. Participants were allowed to have received bisphosphonates (IV or oral) if they were indicated for osteoporosis and had been stopped by the time the subject received the first study drug. A planned dental procedure was an exclusion criterion. Calcium and vitamin D use were strongly encouraged. SREs were assessed by skeletal surveys of the skull, spine, chest, pelvis, arm from shoulder to elbow, and leg from hip to knee at baseline and every 12 weeks thereafter. The primary endpoint was time to first SRE. Study discontinuation occurred when patients received open-label IV or oral bisphosphonate therapy.
A total of 950 men were assigned to the denosumab arm, while 951 men were assigned to the zoledronic acid arm. At the time of the primary analysis cut-off, the median time on study for the denosumab group was 12.2 months, and the median time on study for the zoledronic acid group was 11.2 months. Approximately 3% of each group had received prior oral bisphosphonate therapy. Approximately one-third of patients in each arm were receiving docetaxel-based chemotherapy.
Time to first on-study SRE was longer in the denosumab group than in the zoledronic acid group: 20.7 months vs 17.1 months (hazard ratio [HR], 0.82; 95% confidence interval [CI], 0.71–0.95; P = .0002 for the noninferiority analysis and .008 for the superiority analysis). There were 386 events (41%) in the zoledronic acid arm and 341 events (36%) in the denosumab arm. Most of the events were instances of palliative radiation (21% vs 19%) and pathologic fracture (15% vs 14%). Spinal cord compression occurred in 4% of patients receiving zoledronic acid and in 3% of those receiving denosumab. Surgery to the bone occurred in < 1% of patients in each group.
Overall survival, prostate cancer progression, and PSA changes were similar between the two groups. Markers of bone turnover-urinary N-telopeptide and serum bone-specific alkaline phosphatase-were suppressed to a greater extent in the denosumab group. No neutralizing antibodies to denosumab were detected.
Adverse events were similar between the groups, except that hypocalcemia occurred more frequently in the denosumab group. Grade 3+ hypocalcemia occurred in 5% of those receiving denosumab, compared with 1% of those receiving zoledronic acid. ONJ occurred in 1% of patients in the zoledronic acid arm and in 2% of those in the denosumab arm during the first year, but the occurrence of ONJ appeared to increase with prolonged follow-up in both arms.[17] New cancer diagnoses were made in 2% of the patients in the denosumab arm and in 1% of those in the zoledronic acid arm. There were protocol-mandated dose adjustments for changes in creatinine level in 22% of the patients in the zoledronic acid arm, and 15% had doses withheld. In the denosumab arm, no doses were missed for renal dysfunction.
There were more Common Terminology Criteria for Adverse Events (CTCAE) grade 3 or 4 adverse events in the denosumab arm (72%, compared with 66% in the zoledronic acid arm). However, the treatment discontinuation rates were similar (17% vs 15% for the denosumab and zoledronic acid arms, respectively), suggesting similar tolerability.
There have been subsequent studies of patients with poor renal function who were treated with denosumab. Extension trials of denosumab have examined its effect in osteoporotic women with impaired renal function and found that there does not appear to be an increased risk of renal dysfunction.[18] In one cohort, 73 women with an estimated GFR of 15–29 mL/min were given denosumab, 60 mg every 6 months for 3 years. This group did not develop worse renal function, but there were too few events to find a significant benefit. Also with regard to safety, a recent systematic review that included all patients who participated in clinical trials of solid tumors estimated the incidence of ONJ with denosumab at 1.7%.[19] Both zoledronic acid and denosumab are in use today in prostate cancer patients.
Enzalutamide
Enzalutamide is a pure androgen receptor antagonist. The dose is 160 mg orally once a day.[20] The AFFIRM study randomized, in a 2:1 fashion, 1,199 patients with mCRPC who had previously received chemotherapy to treatment with enzalutamide, 160 mg orally per day, or to placebo.[21] It found that enzalutamide improved overall survival and radiographic progression-free survival, and also prolonged the time to SRE.
During the study, 448 of all participants (37%) had at least one SRE. There were 287 SREs (36%) in the enzalutamide-treated group vs 161 (40%) in the placebo group.[22] The most common SRE in both groups was radiation to the bone (22% in the enzalutamide group and 25% in the placebo group), followed by spinal cord compression (8% and 7%), clinically apparent pathologic bone fracture (4% and 4%), change of antineoplastic therapy (3% and 4%), and surgery to the bone (1% and < 1%). The median time to first SRE was 16.7 months in the enzalutamide group vs 13.3 months in the placebo group (HR, 0.69; 95% CI, 0.57–0.84; P = .0001).
Approximately half of patients were receiving bisphosphonate therapy at baseline. For those on a bisphosphonate, use of enzalutamide did not statistically increase time to first SRE (HR, 0.762; 95% CI, 0.577–1.007; P = .553). For those not on a bisphosphonate, enzalutamide did increase time to first SRE, (HR, 0.614; 95% CI, 0.465–0.811; P = .0005). However, the study was not designed or powered for these exploratory comparisons of treatment effect by bisphosphonate exposure.
The number of adverse events in the enzalutamide arm was similar to the number in the placebo arm. However, there were more instances of fatigue, diarrhea, hot flashes, musculoskeletal pain, and headache in the enzalutamide group. There were six seizures in the enzalutamide group, but many of those who had a seizure were felt to have been predisposed to seizure due to a comorbid condition or concomitant medication. The rate of treatment discontinuation for an adverse event was slightly higher in the placebo group.
The PREVAIL study randomized 1,717 men with chemotherapy-naive mCRPC 1:1 to enzalutamide vs placebo.[23] This study also showed improvement in overall survival and radiographic progression-free survival. Participants were permitted to receive bisphosphonate therapy as long as it had been started at least 4 weeks prior to study entry, and bisphosphonates could be started during the study. Unlike in AFFIRM, denosumab was not specifically mentioned in the protocol, but would fall under “other approved bone-targeting agents,” which were permitted. Median time to first SRE was similar in the two groups, but the risk of a first SRE was decreased in the enzalutamide group: 32% vs 37% (HR, 0.72; P < .0001). Median time to first SRE was 31.1 months (95% CI, 29.5 months–not reached) in the enzalutamide group and 31.3 months (95% CI, 23.9 months–not reached) in the placebo group (HR, 0.72; 95% CI, 0.61–0.84; P < .0001).[24]
Abiraterone
Abiraterone is an inhibitor of CYP17, a critical enzyme in steroid synthesis. When CYP17 is blocked, conversion from mineralocorticoids to glucocorticoids and sex hormones is inhibited.[25] Concurrent prednisone helps correct the mineralocorticoid excess and glucocorticoid deficiency that is caused by this blockage, and prednisone itself has anticancer properties. The recommended regimen is abiraterone, 1,000 mg orally once a day, plus prednisone, 5 mg orally twice a day.[11]
From May 2008 to July 2009, the COU-AA-301 study enrolled 1,195 patients to compare the safety and efficacy of abiraterone plus prednisone (n = 797) against placebo plus prednisone (n = 398) in men with mCRPC after exposure to docetaxel chemotherapy.[26] This study found improved overall survival, time to PSA progression, progression-free survival, and PSA response rate in the abiraterone group relative to the placebo group.
Nearly 90% of study participants had bone metastases at diagnosis, and pain at baseline was not different between groups.[27] There were 235 SREs (29%) in the abiraterone group and 130 (33%) in the placebo group. However, the time to first SRE was longer in the abiraterone group than in the placebo group: 25.0 months vs 20.3 months (HR, 0.615; 95% CI, 0.475–0.791; P = .0001). The most common SRE was radiation to the bone (24% in the abiraterone group vs 46.1% in the placebo group); others included pathologic fracture (6.0% vs 4.0%), surgery to the bone (1.7% vs 1.0%), and spinal cord compression (7.3% vs 14.0%). Of patients in the abiraterone group, 44.9% received concurrent bisphosphonate treatment, compared with 50.0% in the placebo group (P = .0816).
Toxicities related to mineralocorticoid excess were more common in the abiraterone group: fluid retention and edema (31% in the abiraterone group vs 22% in the placebo group) and hypokalemia (17% vs 8%). Rates of other toxicities were similar in the two groups. Transaminase elevation was seen in both groups, and it is recommended that results of liver function tests be monitored closely. Cardiac events were slightly higher in the abiraterone arm (13% vs 11%). Discontinuation of abiraterone for toxicity was described as “low.”
Studies of enzalutamide and abiraterone demonstrated for the first time that potent androgen receptor–targeted antineoplastic therapy has a direct impact on the skeletal complications of prostate cancer.
Radium-223
Radium-223 is a radioisotope administered IV every 4 weeks at 50 kBq/kg for 6 treatments.[16] Radium has the same valence as calcium and is processed in the bone like calcium. Other approved radiopharmaceuticals (strontium, samarium, rhenium) are beta-emitting; radium-223 is the only alpha-emitting radiopharmaceutical in use. Alpha particles are much heavier than beta particles and therefore cause a far greater number of double-strand DNA breaks per particle. They do not travel as far as beta particles and appear to cause less myelosuppression,[28] but there have been no head-to-head comparisons between radium-223 and other radiopharmaceuticals.
The ALSYMPCA trial randomized 921 patients with mCRPC from June 2008 to February 2011 in a 2:1 fashion to IV radiation with the alpha-emitter radium-223 or placebo.[29] Participants had to have two or more bone metastases and no known visceral disease or enlarged lymph nodes > 3 cm in the short axis. They had to have received docetaxel or declined docetaxel treatment. They also had to have had bone pain requiring analgesic medication or treatment with external beam radiation therapy within the previous 12 weeks. They had to have adequate liver and hematologic function, where the latter was defined as an absolute neutrophil count of at least 1.5 × 109 cells/L and a platelet count of at least 100 × 109 cells/L. Exclusion criteria included hemibody external beam radiation therapy, systemic radiotherapy within the past 24 weeks, blood transfusion or use of erythropoietin-stimulating agents within 4 weeks, imminent cord compression, or unmanageable fecal incontinence.
In this study, overall survival was the primary outcome. Secondary endpoints were PSA progression-free survival, time to an increase in alkaline phosphatase, total alkaline phosphatase response, time to first SSE (external beam radiation for bone pain, occurrence of new symptomatic pathologic bone fracture, spinal cord compression, or tumor-related orthopedic surgical intervention), and time to each SSE.
Of the 614 patients who received radium-223, 262 (43%) received concomitant bisphosphonates from the first injection until 12 weeks after the last dose.[30] Of the 307 patients in the placebo arm, 132 (43%) were receiving bisphosphonate therapy. Fifty-seven percent of participants in each group had received prior docetaxel chemotherapy.
Men who received radium-223 (n = 614) lived longer than those who received placebo (n = 307); (HR for death, 0.70; 95% CI, 0.55–0.88; P = .002). Time to SSE was a secondary endpoint. There were 202 patients (33%) in the radium arm and 116 (38%) in the placebo arm who had at least one SSE. Time to first SSE was longer in the radium group than in the placebo group: 15.6 months vs 9.8 months (HR, 0.66; 95% CI, 0.52–0.83; P = .00037). This interval was longer even when participants were receiving a bisphosphonate at baseline (HR, 0.49; 95% CI, 0.33–0.74; P = .00048) or had received previous docetaxel (HR, 0.62; 95% CI, 0.46–0.82; P = .00087).
External beam radiation therapy for a painful osseous metastasis was the most common SSE in both groups: 30% in the radium-223 group and 34% in the placebo group. The median time to this event was 17.1 months vs 17.5 months, respectively (HR, 0.67; 95% CI 0.53–0.85; P = .00117). There was no difference in time to symptomatic pathologic bone fracture (HR, 0.62;, 95% CI, 0.35–1.09; P = .10) or time to tumor-related orthopedic surgical intervention (HR, 0.72; 95% CI, 0.28–1.82; P = .48), but the study was not powered for these endpoints. There was improvement in the time to spinal cord compression in the radium-223 group compared with the placebo group (HR, 0.52;95% CI, 0.29–0.93; P = .03). The rate of symptomatic bone fracture was 5% in the radium group and 7% in the placebo group. The rates of spinal cord compression were 4% and 7%, respectively, and the rate of orthopedic surgical intervention was 2% in each group.
The chief toxicities in this study were thrombocytopenia (12% in the radium group vs 6% in the placebo group) and diarrhea (25% vs 15%). There were more treatment discontinuations for toxicities in the placebo group (16% in the radium group vs 21% in the placebo group).
The ALSYMPCA trial was the first demonstration of a radiopharmaceutical modifying the natural history of advanced prostate cancer beyond the modulation of pain.
Cost-Effectiveness
As described above, denosumab reduces SREs to a greater extent than zoledronic acid does, but it is also more expensive and has a somewhat different safety profile. Several papers have described the cost-effectiveness of these agents, and they have come to different conclusions. However, many of these analyses included authors from Amgen (the maker of denosumab) and Novartis (the maker of zoledronic acid). One group of authors who performed a systematic review, including all patients with osseous metastatic disease from solid tumors, found that quality-of-life improvements were similar in the two groups and that denosumab was not cost-effective.[31] According to this review, 1 year of denosumab cost $35,341 and 1 year of zoledronic acid cost $27,528.
Conclusions
Five agents have now been shown to decrease the burden of skeletal complications in mCRPC with osseous metastatic disease. Despite these advances, SREs remain common, still affecting around 33% of patients, even in clinical trials.
Two of the agents described above are used in conjunction with an antineoplastic. Neither denosumab nor zoledronic acid has shown a clinically relevant anticancer effect. A treating clinician could use either of these agents together with an antineoplastic agent, such as sipuleucel-T, enzalutamide, abiraterone, docetaxel, cabazitaxel, or radium-223. The incidence of skeletal complications probably justifies the concurrent use of antineoplastic therapies that reduce SREs along with bone-specific agents, but it is important to recognize that no prospective study has evaluated the use of these drugs in combination to determine the incremental value of adding one to the other.
The optimal duration of treatment with denosumab and zoledronic acid is unclear because this question has not been addressed directly in clinical trials. In the osteoporosis literature, the clinical benefit is most pronounced during the first 3 years of treatment.[32,33] A recent study of zoledronic acid, 5 mg IV, given one time in osteoporosis, showed that the antiresorptive effect persisted for 5 years.[34] In animal models, the half-life of zoledronic acid in the bone is 300 days.[35] Furthermore, prolonged treatment with bisphosphonates is connected to atypical subtrochanteric and diaphyseal femoral fractures. The American Society for Bone and Mineral Research convened to discuss this issue in 2009 and again in 2013.[35] They recommended that treatment with the oral bisphosphonate alendronate be limited to 5 years, in part to help prevent atypical femoral fractures (AFF). As a point of reference, treatment of osteoporosis is weekly with alendronate or once annually with zoledronic acid (5-mg dose). Treatment with zoledronic acid for mCRPC with osseous disease is 4 mg every 4 weeks, which is approximately 10 times the dose of annual zoledronic acid. AFFs are relatively rare (3.2 to 50 cases per 100,000 person-years), but the rate goes up substantially with longer exposure (about 100 per 100,000 person-years).[36] Similarly, the risk of ONJ has been linked to duration and intensity of exposure to antiresorptive agents.[17] It is worth noting that the original study of zoledronic acid in mCRPC limited treatment to 24 months, and the incremental benefit of continuing treatment beyond the initial analysis at 15 months was unclear. In our practice, we limit the use of IV bisphosphonates to 2 years. There are no published recommendations for bisphosphonate use in mCRPC, but there are recommendations in multiple myeloma, for which experts suggest that IV bisphosphonate therapy be limited to 2 years.[37,38]
Denosumab does not reside in the bone the way bisphosphonates do. There are data to suggest that bone density decreases after discontinuation of denosumab and that perhaps this therapy should be used on an ongoing basis.[39] In clinical trials, it has been used for as long as 41 months. However, risks of ONJ do increase with duration of exposure. Thus, the optimal duration of denosumab therapy remains to be defined.
Radium-223 is limited to six treatments, but there are studies evaluating longer treatment durations. Abiraterone and enzalutamide are usually continued for as long as a patient has clinical benefit without significant toxicities.
Radium-223, enzalutamide, and abiraterone are not currently approved for use in castration-sensitive prostate cancer, even if it is metastatic to the bone. Zoledronic acid and denosumab are used in earlier stages of prostate cancer for bone density loss, but the doses are decreased: zoledronic acid is given at a dosage of 5 mg IV once a year or 4 mg IV every 6 months, and denosumab is given at a dosage of 60 mg SQ every 6 months. A recent study examining zoledronic acid at a dosage of 4 mg IV every 4 weeks for castration-sensitive metastatic prostate cancer to the bone did not show increased time to an SRE relative to placebo, and we do not endorse the use of zoledronic acid in castration-sensitive disease.[40]
Financial Disclosure:Dr. Graff receives research funding from Astellas Pharma Global, Bayer, and Medivation. Dr. Beer receives research funding from Astellas Pharma Global, Janssen, and Medivation; he receives consulting fees from Astellas Pharma Global and Bayer.
References:
1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin. 2015;65:5-29.
2. Bubendorf L, Schopfer A, Wagner U, et al. Metastatic patterns of prostate cancer: an autopsy study of 1,589 patients. Hum Pathol. 2000;31:578-83.
3. Coleman RE. Clinical features of metastatic bone disease and risk of skeletal morbidity. Clin Cancer Res. 2006;12:6243s-49s.
4. Mundy GR. Metastasis to bone: causes, consequences and therapeutic opportunities. Nat Rev Cancer. 2002;2:584-93.
5. Oefelein MG, Ricchiuti V, Conrad W, Resnick MI. Skeletal fractures negatively correlate with overall survival in men with prostate cancer. J Urol. 2002;168:1005-7.
6. Roghmann F, Antczak C, McKay RR, et al. The burden of skeletal-related events in patients with prostate cancer and bone metastasis. Urol Oncol. 2015;33:17 e9-e18.
7. Saad F, Gleason DM, Murray R, et al. A randomized, placebo-controlled trial of zoledronic acid in patients with hormone-refractory metastatic prostate carcinoma. J Natl Cancer Inst. 2002;94:1458-68.
8. Fizazi K, Carducci M, Smith M, et al. Denosumab versus zoledronic acid for treatment of bone metastases in men with castration-resistant prostate cancer: a randomised, double-blind study. Lancet. 2011;377:813-22.
9. Cannon J, Silvestri S, Munro M. Imaging choices in occult hip fracture. J Emerg Med. 2009;37:144-52.
10. Helweg-Larsen S, Sorensen PS. Symptoms and signs in metastatic spinal cord compression: a study of progression from first symptom until diagnosis in 153 patients. Eur J Cancer. 1994;30A:396-8.
11. Centocor Orth Biotech Inc. Zytiga (abiraterone acetate): US prescribing information. 2011. Available at: http://accessdata.fda.gov/drugsatfda_docs/label/2012/202379s004lbl.pdf. Accessed May 15, 2015.
12. Cockcroft-Gault equation. Available at: http://www.mdrd.com. Accessed May 15, 2015.
13. Price N, Lipton A, Jain VK, Ruggiero S. Prevention and management of osteonecrosis of the jaw associated with bisphosphonate therapy. Support Cancer Ther. 2004;2:14-7.
14. Abraham I. Intravenous bisphosphonate treatment and osteonecrosis of the jaw in patients with cancer: wide CIs, Yule-Simpson and King Kong effects, and no therapeutic outcomes. J Clin Oncol. 2010;28:e143-4; author reply e45-7.
15. Vahtsevanos K, Kyrgidis A, Verrou E, et al. Longitudinal cohort study of risk factors in cancer patients of bisphosphonate-related osteonecrosis of the jaw. J Clin Oncol. 2009;27:5356-62.
16. Amgen, Inc. Xgeva (denosumab) injection: US prescribing information. 2015. Available at: http://www.accessdata.fda.gov/drugsatafda_docs/label/2015/125320s174lbl.pdf. Accessed May 15, 2015.
17. Lipton A, Saad F, Van Poznak CH, et al. Incidence of osteonecrosis of the jaw in patients receiving denosumab or zoledronic acid for bone metastases from solid tumors or multiple myeloma: results from three phase III trials. J Clin Oncol. 2013;31(suppl); abstr 9640:
18. Jamal SA, Ljunggren O, Stehman-Breen C, et al. Effects of denosumab on fracture and bone mineral density by level of kidney function. J Bone Miner Res. 2011;26:1829-35.
19. Boquete-Castro A, Gomez-Moreno G, Calvo-Guirado JL, et al. Denosumab and osteonecrosis of the jaw. A systematic analysis of events reported in clinical trials. Clin Oral Implants Res. 2015 Feb 2. [Epub ahead of print]
20. Astellas Pharma US, Inc. Xtandi (enzalutamide) oral capsules: US prescribing information. 2012. Available at: http://www.accessdata.fda.gov/drugsatfda_docs/label/2014/203415s003lbl.pdf. Accessed May 15, 2015.
21. Scher HI, Fizazi K, Saad F, et al. Increased survival with enzalutamide in prostate cancer after chemotherapy. N Engl J Med. 2012;367:1187-97.
22. Fizazi K, Scher HI, Miller K, et al. Effect of enzalutamide on time to first skeletal-related event, pain, and quality of life in men with castration-resistant prostate cancer: results from the randomised, phase 3 AFFIRM trial. Lancet Oncol. 2014;15:1147-56.
23. Beer TM, Armstrong AJ, Rathkopf DE, et al. Enzalutamide in metastatic prostate cancer before chemotherapy. N Engl J Med. 2014;371:424-33.
24. Loriot Y, Miller K, Sternberg CN, et al. Effect of enzalutamide on health-related quality of life, pain, and skeletal-related events in asymptomatic and minimally symptomatic, chemotherapy-naive patients with metastatic castration-resistant prostate cancer (PREVAIL): results from a randomised, phase 3 trial. Lancet Oncol. 2015;16:509-21.
25. Attard G, Reid AH, Auchus RJ, et al. Clinical and biochemical consequences of CYP17A1 inhibition with abiraterone given with and without exogenous glucocorticoids in castrate men with advanced prostate cancer. J Clin Endocrinol Metab. 2012;97:507-16.
26. de Bono JS, Logothetis CJ, Molina A, et al. Abiraterone and increased survival in metastatic prostate cancer. N Engl J Med. 2011;364:1995-2005.
27. Logothetis CJ, Basch E, Molina A, et al. Effect of abiraterone acetate and prednisone compared with placebo and prednisone on pain control and skeletal-related events in patients with metastatic castration-resistant prostate cancer: exploratory analysis of data from the COU-AA-301 randomised trial. Lancet Oncol. 2012;13:1210-7.
28. Ritter MA, Cleaver JE, Tobias CA. High-LET radiations induce a large proportion of non-rejoining DNA breaks. Nature. 1977;266:653-5.
29. Parker C, Nilsson S, Heinrich D, et al. Alpha emitter radium-223 and survival in metastatic prostate cancer. N Engl J Med. 2013;369:213-23.
30. Sartor O, Coleman R, Nilsson S, et al. Effect of radium-223 dichloride on symptomatic skeletal events in patients with castration-resistant prostate cancer and bone metastases: results from a phase 3, double-blind, randomised trial. Lancet Oncol. 2014;15:738-46.
31. Ford J, Cummins E, Sharma P, et al. Systematic review of the clinical effectiveness and cost-effectiveness, and economic evaluation, of denosumab for the treatment of bone metastases from solid tumours. Health Technol Assess. 2013;17:1-386.
32. Bone HG, Chapurlat R, Brandi ML, et al. The effect of three or six years of denosumab exposure in women with postmenopausal osteoporosis: results from the FREEDOM extension. J Clin Endocrinol Metab. 2013;98:4483-92.
33. Black DM, Reid IR, Boonen S, et al. The effect of 3 versus 6 years of zoledronic acid treatment of osteoporosis: a randomized extension to the HORIZON-Pivotal Fracture Trial (PFT). J Bone Miner Res. 2012;27:243-54.
34. Grey A, Bolland MJ, Horne A, et al. Five years of anti-resorptive activity after a single dose of zoledronate-results from a randomized double-blind placebo-controlled trial. Bone. 2012;50:1389-93.
35. Fleisch H. Bisphosphonates. Pharmacology and use in the treatment of tumour-induced hypercalcaemic and metastatic bone disease. Drugs. 1991;42:919-44.
36. Shane E, Burr D, Abrahamsen B, et al. Atypical subtrochanteric and diaphyseal femoral fractures: second report of a task force of the American Society for Bone and Mineral Research. J Bone Miner Res. 2014;29:1-23.
37. Lacy MQ, Dispenzieri A, Gertz MA, et al. Mayo Clinic consensus statement for the use of bisphosphonates in multiple myeloma. Mayo Clin Proc. 2006;81:1047-53.
38. Kyle RA, Yee GC, Somerfield MR, et al. American Society of Clinical Oncology 2007 clinical practice guideline update on the role of bisphosphonates in multiple myeloma. J Clin Oncol. 2007;25:2464-72.
39. Bone HG, Bolognese MA, Yuen CK, et al. Effects of denosumab treatment and discontinuation on bone mineral density and bone turnover markers in postmenopausal women with low bone mass. J Clin Endocrinol Metab. 2011;96:972-80.
40. Smith MR, Halabi S, Ryan CJ, et al. Randomized controlled trial of early zoledronic acid in men with castration-sensitive prostate cancer and bone metastases: results of CALGB 90202 (Alliance). J Clin Oncol. 2014;32:1143-50.
41. Saad F, Gleason DM, Murray R, et al. Long-term efficacy of zoledronic acid for the prevention of skeletal complications in patients with metastatic hormone-refractory prostate cancer. J Natl Cancer Inst. 2004;96:879-82.
2 Commerce Drive
Cranbury, NJ 08512
All rights reserved.