Releasing the Brake on the Immune System: The PD-1 Strategy for Hematologic Malignancies
Incorporation of PD-1 blockade into the treatment algorithms for hematologic malignancies is currently being pursued in multiple active clinical trials. Here we review the data on anti–PD-1 monoclonal antibodies to date and discuss ongoing and future clinical trials.
Figure 1: Checkpoint Inhibition via the PD-1 Pathway ‘Puts the Brakes on’ the Antitumor Response, While PD-1– or PD-L1–Blocking Antibodies Release the Brake
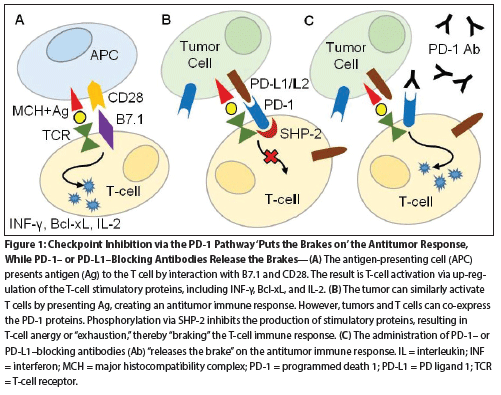
Figure 2: IHC Staining for PD-1
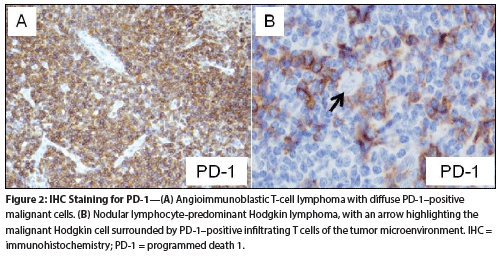
Table 1: Agents Targeting the PD-1 Pathway in Hematologic Malignancies
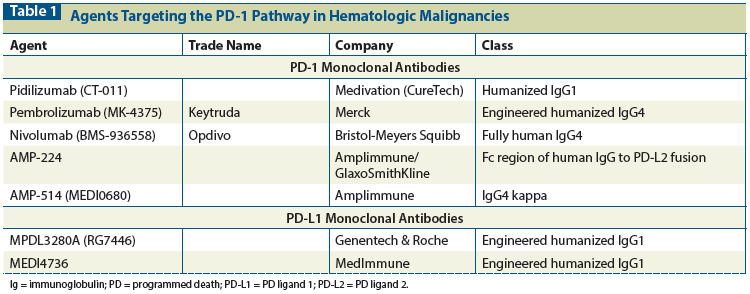
Table 2: Clinical Trial Results With PD-1 Antibodies in Hematologic Malignancies
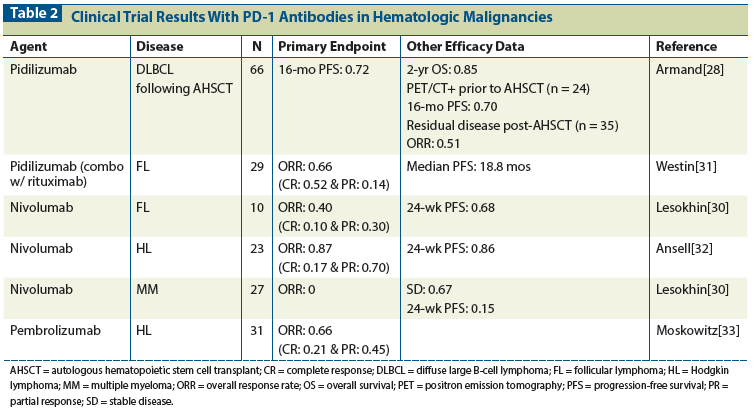
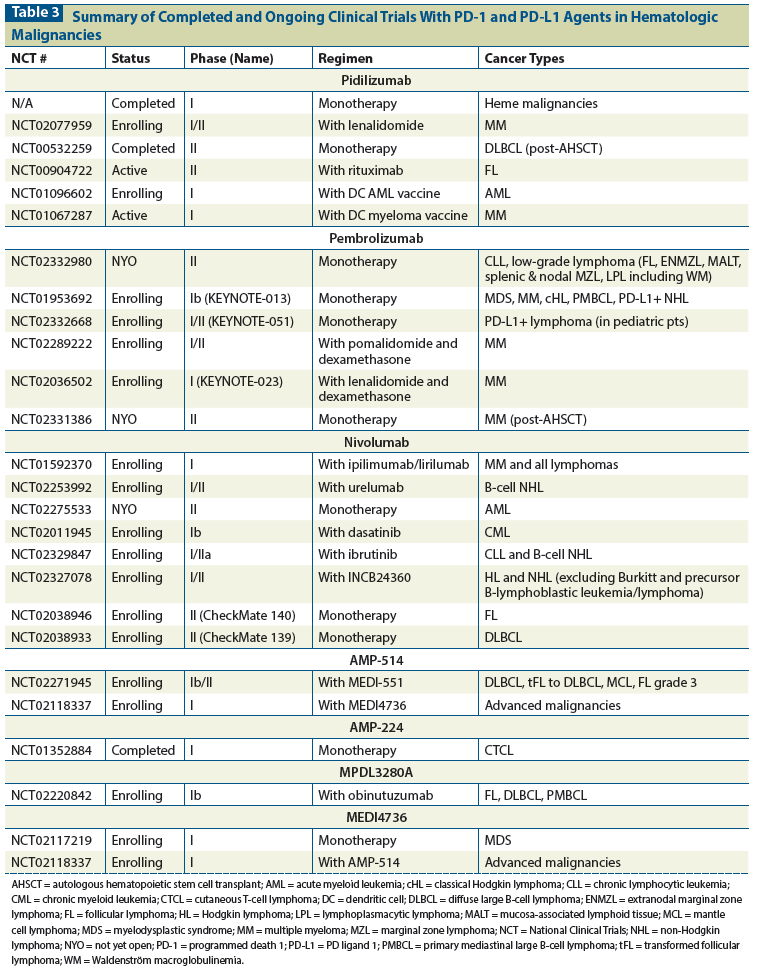
Table 3: Summary of Completed and Ongoing Clinical Trials With PD-1 and PD-L1 Agents in Hematologic Malignancies
Manipulation of the immune system as a viable cancer treatment strategy has re-emerged. The programmed death 1 (PD-1) pathway is an important, physiologic immune checkpoint necessary to limit autoimmune processes but co-opted by tumors to suppress the antitumor response and allow tumor escape. Blockade of the PD-1 pathway through the use of PD-1 or PD ligand 1(PD-L1) antibodies releases this brake on the immune response. The anti–PD-1 antibodies have produced encouraging results across a broad range of malignancies. Many hematologic malignancies have usurped the PD-1 pathway. Recent investigations have explored the use of anti–PD-1 therapy in hematologic malignancies, with encouraging results. Incorporation of PD-1 blockade into the treatment algorithms for hematologic malignancies is currently being pursued in multiple active clinical trials. Here we review the data on anti–PD-1 monoclonal antibodies to date and discuss ongoing and future clinical trials.
Introduction
The programmed death 1 (PD-1) pathway has emerged as a mechanism for immune tolerance. Expression of PD-1 or the PD-1 ligands, PD-L1 and PD-L2, has been identified both on tumor cells and within the tumor microenvironment. Through the PD-1 pathway, tumor cells are able to suppress the antitumor immune response via T-cell anergy or “exhaustion.” A new class of immunotherapy agents includes anti–PD-1 and anti–PD-L1 monoclonal antibodies, which have demonstrated remarkable responses across a variety of solid and hematologic malignancies. Manipulation of the PD-1 pathway continues to be an area of great interest as a strategy for circumventing tumor escape from the immune response.
Manipulation of the Immune System
The field of cancer therapeutics has shown an increasing interest in harnessing the immune system as a treatment approach. The development of monoclonal antibodies and their use as targeted therapy has greatly altered cancer treatment algorithms. Monoclonal antibodies have been developed for use in a broad array of therapeutic strategies, including induction of cell-specific apoptosis, blockade of targeted molecular functions, and modulation of cell signaling pathways.[1] Monoclonal antibodies are associated with less toxicity than cytotoxic chemotherapy. Recently, investigators have focused on immune checkpoints, key elements in the physiologic process that limits autoimmunity in the normal host but that also limits immune surveillance in cancer, allowing tumor escape. Immune checkpoints include inhibitory pathways of the immune system that maintain self-tolerance through regulation of the immune response.[2] By “releasing the brakes” on the immune system, the antitumor responses can be restored.
One such immune checkpoint is the PD-1 pathway. The PD-1 protein was first described in the early 1990s and was initially thought to play a role in programmed cell death or apoptosis.[3] Further investigation, however, revealed a role in manipulation of T-cell subsets. Ultimately, the PD-1 pathway was found to play a role in regulation of T-cell activation and in apoptotic pathways of effector/memory T lymphocytes. Binding of the PD-1 receptor with PD-L1 interferes with T-cell receptor signaling and blocks phosphatidylinositol 3-kinase (PI3K) activation. Blocking of PI3K activation leads to down-regulation of stimulatory proteins required for T-cell proliferation.[4,5] Checkpoint inhibition via the PD-1 pathway suppresses the antitumor response (Figure 1). The PD-1 pathway has been adapted by multiple pathologic processes-including chronic infections such as HIV, hepatitis, and parasitic infections-and autoimmune diseases such as rheumatoid arthritis, inflammatory bowel disease, and diabetes mellitus type 1.[6-9] Co-optation of the PD-1 pathway by malignant cells was first described in the early 2000s, leading to investigations into PD-1 blockade as a possible mechanism for cancer therapy.[10,11]
The PD-1 Pathway in Hematologic Malignancies
PD-1 pathway expression markers, including PD-1, PD-L1, and PD-L2, have been confirmed in multiple hematologic malignancies. Marker expression is characterized by immunohistochemistry (IHC) and can be present either on tumor cells or on the infiltrating lymphocytes within the surrounding tumor microenvironment (Figure 2). Evidence for the PD-1 pathway, as demonstrated by IHC, has been found in multiple myeloma, acute myeloid leukemia, Hodgkin lymphoma, and the non-Hodgkin lymphomas, including both B-cell and T-cell subtypes.[12-15] PD-1 marker expression in non-Hodgkin lymphomas has been described in chronic lymphocytic leukemia/small lymphocytic lymphoma, follicular lymphoma, diffuse large B-cell lymphoma (DLBCL), primary mediastinal B-cell lymphoma, anaplastic large-cell lymphoma, and angioimmunoblastic T-cell lymphoma.[13,16-18] There is heterogeneity of PD-1 marker expression even within specific lymphoma subtypes.
PD-1 marker expression has been explored as a potential predictor of prognosis. For solid tumors, the expression of PD-L1 has been correlated with poorer clinical outcomes, including poor prognosis, decreased survival, increased tumor size and stage, and increased tumor aggressiveness.[19] For hematologic malignancies, particularly lymphomas, the association of PD-1 marker expression and clinical outcomes has been variable across tumor subtypes. The expression of PD-1 by classical Hodgkin lymphoma is a stage-independent negative prognostic factor for overall survival (OS).[20] One retrospective analysis in follicular lymphoma found that PD-1 positivity was an independent poor prognostic factor.[21] Another study in follicular lymphoma found that expression of PD-1 on tumor-infiltrating lymphocytes was associated with improvements in progression-free survival (PFS) and OS.[22]
Recent research has identified a particular genetic mutation leading to increased PD-L1 and PD-L2 expression in classical Hodgkin lymphoma, nodular sclerosis type. Amplification at chromosome 9p24.1 results in overexpression of the PD-1 ligands by the malignant Reed-Sternberg cells.[23] This amplification at chromosome 9p24.1 is the only identified genetic alteration associated with the PD-1 markers and appears to be specific to Hodgkin lymphoma. Extended chromosome 9p24.1 amplification also includes a Janus kinase 2 (JAK2) locus that increases protein expression and activity. Amplifications in chromosome 9p24.1 for PD-L1 expression and amplifications in chromosome 2q37.3 for PD-1 expression may be relevant sites of genetic variability to explore in other hematologic malignancies.[24,25]
PD-1 and PD-L1 Agents
There are currently seven monoclonal antibodies targeting the PD-1 pathway in clinical trials in hematologic malignancies: five that target PD-1 and two that target PD-L1 (Table 1). The four anti–PD-1 agents vary slightly in composition and in how they were developed. Pidilizumab was the first agent used in hematologic malignancies and was generated through the immunization of BALB/c mice with membranes of a human B-cell lymphoma cell line to form a humanized immunoglobulin (Ig) G1 kappa antibody.[26] The two more widely marketed anti–PD-1 agents that have been approved by the US Food and Drug Administration (FDA) for use in melanoma and squamous non–small-cell lung cancer are pembrolizumab and nivolumab. Both agents are IgG4 isotype antibodies. One agent, AMP-224, is a fusion protein that joins IgG1 with PD-L2 to target PD-1 binding. The Ig backbone varies across these agents, although it likely plays a limited role in the overall efficacy of therapy. Arguments can be made for the superiority of the binding ability of each of these agents to its respective target, but no agent has emerged as a “more potent” therapy. The two PD-L1 monoclonal antibodies also vary slightly, with MPDL3280A having been developed containing an optimized Fc region, and MEDI4736 having an IgG kappa isotype. One proposed limitation of the PD-L1 monoclonal antibodies is that the PD-1 pathway may remain stimulated through alternative expression and binding via the other PD-1 ligand, PD-L2. All antibodies have weight-based dosing, with differing dose levels and schedules.
Clinical Experience
The use of anti–PD-1 therapy in hematologic malignancies is limited to early-phase clinical trials. Most trials include multiple tumor types. Unlike in trials of PD-1 blockade in solid tumors, the eligibility for clinical trial enrollment for hematologic malignancies has not required confirmation of PD-1 or PD-L1 expression by IHC staining. The characterization of PD-1 expression has been more difficult in hematologic malignancies. There is heterogeneity in lymph node involvement for lymphomas, and a lack of a reliable biomarker in circulating diseases like leukemia. The published results from available clinical trials are summarized in Table 2. Here we discuss the available clinical experience for several major disease subsets.
Diffuse large B-cell lymphoma
The initial small phase I trial with pidilizumab included two patients with DLBCL; however, neither had a clinical response with treatment.[27] The results from a larger, phase II, international open-label trial conducted in patients with DLBCL following autologous hematopoietic stem cell transplant (AHSCT) were published in 2013.[28] The trial also included a few patients with primary mediastinal B-cell lymphoma and a few with transformed indolent B-cell non-Hodgkin lymphoma. A total of 72 patients, with a median age of 57, were enrolled and treated with pidilizumab consolidation following AHSCT.
Pidilizumab, 1.5 mg/kg q42d × 3 cycles, was administered 30 to 90 days after AHSCT. Disease assessments with CT imaging were performed at enrollment; prior to cycles 2 and 3; and then at 30, 44, and 69 weeks after the initial pidilizumab dose. Additionally, patients had fluorodeoxyglucose (FDG)–positron emission tomography (PET)/CT imaging at the treating physician’s discretion. The primary objective was PFS at 16 months after the initial pidilizumab dose. The secondary objectives included other important clinical parameters, such as safety and toxicity, and OS.
There were 66 evaluable patients in the trial who completed all 3 cycles of pidilizumab. The observed 16-month PFS was 0.72 (90% confidence interval [CI], 0.6–0.82), and this reached statistical significance when compared with historical controls. The OS at 2 years was 0.85 (90% CI, 0.74–0.92). For the 31 patients with a complete remission prior to AHSCT (as confirmed by a negative FDG-PET/CT scan), the 16-month PFS was 0.72 (90% CI, 0.56–0.84). For the 24 patients with persistent residual disease prior to AHSCT (as confirmed by a positive FDG-PET/CT scan), the 16-month PFS was 0.70 (90% CI, 0.51–0.82). A positive FDG-PET/CT prior to AHSCT is associated with poorer outcomes, with a reported 18-month PFS of 0.52 (90% CI, 0.39–0.63) in patients who did not receive pidilizumab.[29] The improvement in PFS in this cohort suggests that anti–PD-1 therapy may overcome the poorer prognostic implications of a positive FDG-PET/CT scan prior to AHSCT. In the trial, 35 patients (53%) had residual disease following AHSCT by CT imaging. In this cohort, use of pidilizumab resulted in a 51% overall response rate (ORR), including 12 complete responses (CR; 34%) and 6 partial responses (PR; 17%), demonstrating clinical activity of pidilizumab in patients with DLBCL.
The ongoing phase I trial of nivolumab in hematologic malignancies includes 11 patients with heavily pretreated relapsed/refractory DLBCL. The preliminary results were reported in abstract form at the 2014 American Society of Hematology (ASH) Annual Meeting.[30] Patients were treated with nivolumab, 3 mg/kg q2wk, with objective responses observed in four patients (36%), including one patient with a CR (9%), three patients with PRs (27%), and an additional three patients with stable disease (27%). The PFS at 24 weeks was 24%. The results have led to a phase II trial (CheckMate 139; NCT02038933) that is currently enrolling patients.
Follicular lymphoma
The initial small phase I trial with pidilizumab included a single patient with previously untreated follicular lymphoma who achieved a CR.[27] The results of a larger, phase II, open-label, nonrandomized single-institution trial conducted in patients with follicular lymphoma were published in 2014.[31] A total of 30 patients, with a median age of 61, were enrolled and treated with the combination of pidilizumab and rituximab. Patients had relapsed/refractory follicular lymphoma, grade 1 to 2, previously treated with one to four lines of therapy. All patients had previously received rituximab, either as monotherapy or in combination with another agent. The treatment schedule included pidilizumab, 3 mg/kg q28d × 4 cycles. Rituximab, 375 mg/m2, was given once weekly for 4 weeks, starting 17 days following the initial dose of pidilizumab. Patients with stable or improved disease could receive up to 8 additional doses of pidilizumab on the same q28d cycle. CT imaging and bone marrow biopsies were used to assess disease response, and FDG-PET/CT imaging was obtained at the discretion of the treating physician. Disease assessments were performed at enrollment, following cycles 2 and 4, then every 12 weeks through 2 years. The primary objective was objective response rate, which included both complete and partial responses. The secondary objectives included other important clinical parameters, such as safety and toxicity, PFS, and immunologic effects.
There were 29 evaluable patients in the trial, 19 of whom (66%) achieved an objective response; these responses included 15 CRs (52%) and 4 PRs (14%). Of note, 25 patients (86%) had tumor regression. Patients received a median number of 10 cycles of pidilizumab (range, 1–12), and 29 (97%) completed all 4 rituximab infusions. The median follow-up of patients in the trial was 15.4 months. The median time to response was 88 days, with 6 patients (21%) having a substantially delayed time to response of more than 4 months following the initial dose of pidilizumab. The observed median PFS was 18.8 months (95% CI, 14.7–not reached) for all patients, not reached for 19 responders (95% CI, 18.8 to not reached), and 19.6 months (95% CI, 17.5–not reached) for patients with tumor regression on treatment. Overall, the PFS appears improved when compared with the estimated 17.8 months reported in patients treated with rituximab monotherapy.
The ongoing phase I trial with nivolumab in hematologic malignancies includes 10 patients with heavily pretreated relapsed/refractory follicular lymphoma. The preliminary results were reported in abstract form at the 2014 ASH Annual Meeting.[30] Patients were treated with nivolumab, 3 mg/kg q2wk, with objective responses observed in four patients (40%), including one patient with a CR (10%), three patients with PRs (30%), and an additional six patients with stable disease (60%). The PFS at 24 weeks was 68%. The results have led to a phase II trial (CheckMate 140; NCT02038946) that is currently enrolling patients.
Hodgkin lymphoma
The initial small phase I trial with pidilizumab included a single patient with relapsed/refractory Hodgkin lymphoma in whom AHSCT had previously failed. The patient had stable disease for approximately 8 months following treatment.[27] The results of a Hodgkin lymphoma cohort from the ongoing phase I, open-label, dose-escalated study of nivolumab were published in late 2014 and simultaneously announced at the ASH Annual Meeting.[32] There were 23 patients in the trial with relapsed/refractory Hodgkin lymphoma who were treated with nivolumab, 3 mg/kg q2wk, until progression or CR, for up to 2 years. The median age was 35. All patients were heavily pretreated, with 87% having received ≥ 3 prior regimens, 78% having received brentuximab, and 78% having undergone AHSCT. Patients were initially assessed with FDG-PET/CT; then evaluated with CT imaging at 4, 8, 16, and 24 weeks; then every 16 weeks. FDG-PET/CT was subsequently used to confirm CR. The primary objective was safety and toxicity. The secondary objectives included the efficacy endpoints of PFS and OS.
Within this cohort of 23 patients, the overall response rate was 0.87 (95% CI, 0.66–0.97), which included 4 with CR (17%), 13 with PR (70%), and the remaining 3 with stable disease (13%). The PFS at 24 weeks was 0.86 (95% CI, 0.62–0.95). The median number of nivolumab doses was 16 (range, 6–37 doses), with a median treatment duration of 36 weeks (range, 13–77 weeks). There were 15 patients (65%) who received > 90% of the intended doses. With a median duration of follow-up of 40 weeks, the median OS had not been reached. Those who discontinued therapy included 6 patients who chose to proceed to stem cell transplant at the time of best overall response. Tumor analysis from 10 patients confirmed increased expression of PD-L1 and PD-L2 in all, with activation through JAK–Signal Transducer and Activator of Transcription (STAT) signaling. The early results from this trial led to a “breakthrough designation” by the FDA for nivolumab in relapsed/refractory Hodgkin lymphoma following treatment with AHSCT and brentuximab.
The preliminary results of a phase Ib trial (KEYNOTE-013) of pembrolizumab in patients with classical Hodgkin lymphoma, nodular sclerosis type, after brentuximab vedotin failure were presented in abstract form at the 2014 ASH Annual Meeting.[33] A total of 31 patients with relapsed/refractory disease were treated with pembrolizumab, 10 mg/kg IV q2wk. Disease response was assessed at 12 weeks, then every 8 weeks on therapy. The median age was 32 and patients were heavily pretreated, with 52% having received ≥ 5 prior therapies. The median duration of follow-up was 153 days. The ORR was 66%, which included 6 patients with CR (21%) and 13 with PR (45%). There were 6 patients with stable disease (21%). Among the enrolled patients, PD-L1 expression was observed in 100% of the evaluable samples.
Other non-Hodgkin lymphomas
Data on anti–PD-1 therapy in other non-Hodgkin lymphomas are quite limited. The initial small phase I trial of pidilizumab included a single patient with anaplastic large-cell lymphoma; however, no response was observed.[28] The ongoing phase I trial of nivolumab in hematologic malignancies reported findings in 13 patients with mycosis fungoides, 5 patients with peripheral T-cell lymphomas, 5 patients with “other” T-cell lymphomas, and 8 patients with “other” B-cell lymphomas. PRs were observed in two patients with mycosis fungoides and two patients with peripheral T-cell lymphomas. There were an additional 15 patients with stable disease.[30] Several interesting clinical trials are currently active. One trial of pembrolizumab (NCT02332980) focuses on patients with low-grade lymphomas and includes marginal zone lymphomas, mucosa-associated lymphoid tissue (MALT) lymphoma, and lymphoplasmacytic lymphomas. Another upcoming trial (NCT02329847) will use a combination of nivolumab and ibrutinib for B-cell non-Hodgkin lymphoma.
Multiple myeloma
The use of PD-1 blockade in multiple myeloma has been explored in several published clinical trials, with overall disappointing results. The small phase I trial of pidilizumab included a single patient with stage I untreated multiple myeloma who received pidilizumab at the highest dose level. The patient had stable disease but no reported response. The ongoing phase I trial of nivolumab in hematologic malignancies includes 27 patients with relapsed/refractory multiple myeloma. The preliminary results were reported in abstract form at the 2014 ASH Annual Meeting.[30] Patients were treated with nivolumab, 3 mg/kg q2wk, with no objective responses observed and 18 patients (67%) with stable disease. The PFS at 24 weeks was 15%. There are multiple ongoing clinical trials for patients with multiple myeloma, including trials of pembrolizumab monotherapy (NCT01953692), the combination of pembrolizumab and lenalidomide (NCT02036502), the combination of pembrolizumab and pomalidomide (NCT02289222), and the combination of pidilizumab and a dendritic cell multiple myeloma vaccine (NCT01067287). The interest in using anti–PD-1 therapy for multiple myeloma is fueled by early mouse models and preclinical studies that established the role of the PD-1 pathway in tumor immune evasion.[10]
Leukemia
The initial small phase I trial of pidilizumab included seven patients with heavily pretreated acute myeloid leukemia, some of whom had received allogeneic stem cell transplantation.[27] Patients were treated with various doses of pidilizumab, but only one patient showed clinical benefit, with a minimal response. The minimal response included independence from platelet transfusions and a reduction in circulating blasts. The patient’s OS was 61 weeks. A clinical trial (NCT01096602) with pidilizumab in combination with a dendritic cell acute myeloid leukemia vaccine is currently enrolling patients. Additionally, clinical trials of nivolumab include monotherapy for acute myeloid leukemia (NCT02275533) and combination therapy with dasatinib for chronic myelogenous leukemia (NCT02011945).
Active Clinical Trials
The use of anti–PD-1 agents is currently a “hot topic” in cancer therapy. We have mentioned several key clinical trials currently enrolling patients. Based on the recent response seen in Hodgkin lymphoma and emerging trends for responses in other diseases, we anticipate that the number of clinical trials of these agents will continue to expand. Table 3 provides a comprehensive list of clinical trials at the time of this publication.
Toxicities With Anti–PD-1 Agents
Anti–PD-1 agents have demonstrated a limited toxicity profile, and overall these agents are well tolerated. When evaluating the side effects observed in the available clinical trials, it is important to realize that patients often have been heavily pretreated for relapsed disease, including, in some cases, prior stem cell transplant. Anti–PD-1 therapy has not been given with cytotoxic chemotherapy in patients with hematologic malignancies. The reported clinical experience is limited to monotherapy and use in combination with rituximab, but new trials are exploring combinations with B-cell receptor inhibitors (eg, ibrutinib).
Pidilizumab has been used exclusively in hematologic malignancies, and the available reported data show grade 3/4 toxicities limited to cytopenias.[27,28,31] The most common grade 1/2 adverse events have included fatigue, upper respiratory tract infection (URI), diarrhea, and cough. A single patient died in the phase II DLBCL trial secondary to disseminated herpes infection, but this was deemed unrelated to pidilizumab.[28] The side effects of nivolumab and pembrolizumab have been best reported in patients with solid tumors. In patients treated with nivolumab for solid malignancies, the most common adverse events of any grade have included pruritis, rash, URI, and peripheral edema. A similar side effect profile was observed when nivolumab was used in patients with hematologic malignancies. The most common serious adverse events seen in patients with B-cell non-Hodgkin lymphoma were pneumonitis (7%) and single cases of possible drug-related myelodysplastic syndromes. A case of pancreatitis was reported in the Hodgkin lymphoma cohort.[30,32] In patients treated for solid malignancies with pembrolizumab, the most common adverse events of any grade included fatigue, nausea, constipation, diarrhea, nonproductive cough, pruritis, rash, anorexia, and arthralgia. The most common grade 3 adverse event was fatigue, which was observed in 7% of patients; otherwise, severe adverse events are considered rare. To date there are no reported toxicity data for the use of pembrolizumab in hematologic malignancies.
There have been no reported grade 5 adverse events with these agents. Immune-mediated reactions, including pneumonitis, colitis, hepatitis, nephritis, and thyroid dysfunction, have been reported in less than 3% of patients with solid malignancies when treated with either pembrolizumab or nivolumab. Importantly, no autoimmune toxicities have emerged in the setting of the treatment of hematologic malignancies, and none were observed with the use of pidilizumab. Nonetheless, the risk of potential immune-mediated toxicities will require close monitoring of patients when using these agents. Important signs and symptoms include diarrhea, severe fatigue, respiratory complaints, liver function test abnormalities, and acute kidney insufficiency. All agents carry a risk of fetal harm, so these drugs should not be used in pregnancy.
Incorporation of PD-1 Blockade Into Treatment Algorithms
With promising response rates demonstrated for several types of relapsed/refractory hematologic malignancies, the incorporation of PD-1 blockade into treatment algorithms needs to be defined. Two main issues arise:
(1) If anti–PD-1 monotherapy is inadequate, will combination with cytotoxic chemotherapy improve response rates with acceptable toxicity?
(2) When combining PD-1 blockade with cytotoxic chemotherapy, should treatment be administered prior to, in combination with, or following chemotherapy?
In hematologic malignancies, the anti–PD-1 antibodies have yet to be combined with cytotoxic chemotherapy. Several clinical trials in solid malignancies are beginning to explore combinations. The ongoing phase I trial for breast and pancreatic cancers (NCT02309177) investigates the use of nivolumab in combination with either nab-paclitaxel, nab-paclitaxel + gemcitabine, or nab-paclitaxel + carboplatin. The primary endpoint is safety and tolerability. A second ongoing phase I clinical trial for non–small-cell lung cancer (CheckMate 012; NCT01454102) includes combinations of nivolumab plus either cisplatin with gemcitabine, cisplatin with pemetrexed, or carboplatin with paclitaxel. Preliminary results reported at the American Society of Clinical Oncology (ASCO) 2014 Annual Meeting revealed no dose-limiting toxicities, but 45% of patients had grade 3/4 adverse events. However, overall there was an acceptable tolerability.[34] Similarly, an ongoing phase I/II trial (KEYNOTE-021; NCT02039674) includes treatment arms using pembrolizumab in combination with either pemetrexed or carboplatin + paclitaxel. A fourth ongoing phase Ib/II trial (PembroPlus; NCT02331251) includes the combination of pembrolizumab with either gemcitabine, irinotecan, or liposomal doxorubicin. The ability to extrapolate from the use of these chemotherapy agents in solid tumors to their use in hematologic malignancies is limited; however, one would anticipate a similarly acceptable toxicity profile.
The timing of anti–PD-1 therapy administration may prove to play a crucial role in maximizing therapeutic benefit. Restoration of the anti-immune response quite simply requires an intact and functional immune system. Use of high-dose cytotoxic chemotherapy certainly dampens the immune response; therefore, administration of chemotherapy may hamper the utility of PD-1 blockade. Several concepts are relevant here and should be considered. Use of anti–PD-1 agents prior to chemotherapy may boost a targeted immune response and heighten the efficacy of subsequent treatment. Administration of anti–PD-1 agents concurrently with chemotherapy likewise may heighten responses, as tumor cell lysis may provide a tumor antigen–rich microenvironment that can prime the immune response. The addition of anti–PD-1 agents following chemotherapy, as in Armand et al,[28] may increase a more robust tumor-specific response during a period of immune reconstitution as the bone marrow recovers from the anticipated myelosuppression associated with cytotoxic agents. These three concepts will require ongoing consideration as subsequent clinical trials are developed.
Conclusion and Future Directions
PD-1 blockade is a very promising treatment strategy for hematologic malignancies. To date, even the limited clinical trial experience has demonstrated a clear therapeutic effect. Despite the heterogeneity of PD-1 marker expression across tumor subtypes, both on malignant cells and within the tumor microenvironment, the PD-1 pathway seems to play a clear role in averting the antitumor response.
Ongoing investigation into the use of anti–PD-1 agents will provide an effective treatment alternative with a well-tolerated side effect profile. The combination of these agents with cytotoxic chemotherapy, targeted agents, or other immunotherapies will require ongoing investigation. Recent research has shown that blockade of the PD-1 pathway can significantly enhance the therapeutic efficacy of chimeric antigen receptor (CAR) T-cell therapy.[35,36] Several proposed benefits for combining anti–PD-1 agents with CAR T-cell therapy include improvement of cell collection due to the boosting of central memory T cells, and maintenance of CAR T-cell proliferation following reinfusion.
As discussed, the adaptation of anti–PD-1 therapies needs to be further explored to determine how these best fit into current treatment algorithms. We anticipate an expansion in the number of clinical trials for hematologic malignancies. Careful clinical trial design to identify the best use of these agents will be crucial. Establishing a biomarker may assist in identifying which tumor subtypes will respond best to therapy. Use of soluble PD-L1 (sPD-L1) may prove to be an effective guide; however, further investigation is necessary to confirm correlations with response.[37] The chromosomal mutations of 9p24.1 in Hodgkin lymphoma have provided an important marker for tumor adaptation. It will be interesting to see whether other genetic alterations are identified in other tumor subtypes. In any event, FDA approval of anti–PD-1 agents in hematologic malignancies is certainly on the horizon.
Acknowledgement:The authors would like to thank Kristy Wolniak, MD, PhD, and Adam Beattie, MD, for the pathology images.
Financial Disclosure:The authors have no significant financial interest in or other relationship with the manufacturer of any product or provider of any service mentioned in this article.
References:
1. Breedveld FC. Therapeutic monoclonal antibodies. Lancet. 2000;355:735-40.
2. Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer. 2012;12:252-64.
3. Ishida Y, Agata Y, Shibahara K, Honjo T. Induced expression of PD-1, a novel member of the immunoglobulin gene superfamily, upon programmed cell death. EMBO J. 1992;11:3887-95.
4. Keir ME, Butte MJ, Freeman GJ, Sharpe AH. PD-1 and its ligands in tolerance and immunity. Annu Rev Immunol. 2008;26:677-704.
5. Okazaki T, Honjo T. The PD-1-PD-L pathway in immunological tolerance. Trends Immunol. 2006;27:195-201.
6. Day CL, Kaufmann DE, Kiepiela P, et al. PD-1 expression on HIV-specific T cells is associated with T-cell exhaustion and disease progression. Nature. 2006;443:350-4.
7. Francisco LM, Sage PT, Sharpe AH. The PD-1 pathway in tolerance and autoimmunity. Immunol Rev. 2010;236:219-42.
8. Lepenies B, Jacobs T. The role of negative costimulators during parasitic infections. Endocr Metab Immune Disord Drug Targets. 2008;8:279-88.
9. Urbani S, Amadei B, Tola D, et al. Restoration of HCV-specific T cell functions by PD-1/PD-L1 blockade in HCV infection: effect of viremia levels and antiviral treatment. J Hepatol. 2008;48:548-58.
10. Iwai Y, Ishida M, Tanaka Y, et al. Involvement of PD-L1 on tumor cells in the escape from host immune system and tumor immunotherapy by PD-L1 blockade. Proc Natl Acad Sci USA. 2002;99:12293-7.
11. Strome SE, Dong H, Tamura H, et al. B7-H1 blockade augments adoptive T-cell immunotherapy for squamous cell carcinoma. Cancer Res. 2003;63:6501-5.
12. Liu J, Hamrouni A, Wolowiec D, et al. Plasma cells from multiple myeloma patients express B7-H1 (PD-L1) and increase expression after stimulation with IFN-{gamma} and TLR ligands via a MyD88-, TRAF6-, and MEK-dependent pathway. Blood. 2007;110:296-304.
13. Shimauchi T, Kabashima K, Nakashima D, et al. Augmented expression of programmed death-1 in both neoplastic and non-neoplastic CD4+ T-cells in adult T-cell leukemia/lymphoma. Int J Cancer. 2007;121:2585-90.
14. Tamura H, Dan K, Tamada K, et al. Expression of functional B7-H2 and B7.2 costimulatory molecules and their prognostic implications in de novo acute myeloid leukemia. Clin Cancer Res. 2005;11:5708-17.
15. Yamamoto R, Nishikori M, Kitawaki T, et al. PD-1-PD-1 ligand interaction contributes to immunosuppressive microenvironment of Hodgkin lymphoma. Blood. 2008;111:3220-4.
16. Dorfman DM, Brown JA, Shahsafaei A, Freeman GJ. Programmed death-1 (PD-1) is a marker of germinal center-associated T cells and angioimmunoblastic T-cell lymphoma. Am J Surg Pathol. 2006;30:802-10.
17. Rosenwald A, Wright G, Leroy K, et al. Molecular diagnosis of primary mediastinal B cell lymphoma identifies a clinically favorable subgroup of diffuse large B cell lymphoma related to Hodgkin lymphoma. J Exp Med. 2003;198:851-62.
18. Xerri L, Chetaille B, Serriari N, et al. Programmed death 1 is a marker of angioimmunoblastic T-cell lymphoma and B-cell small lymphocytic lymphoma/chronic lymphocytic leukemia. Hum Pathol. 2008;39:1050-8.
19. Okazaki T, Honjo T. PD-1 and PD-1 ligands: from discovery to clinical application. Int Immunol. 2007;19:813-24.
20. Muenst S, Hoeller S, Dirnhofer S, Tzankov A. Increased programmed death-1+ tumor-infiltrating lymphocytes in classical Hodgkin lymphoma substantiate reduced overall survival. Hum Pathol. 2009;40:1715-22.
21. Richendollar BG, Pohlman B, Elson P, Hsi ED. Follicular programmed death 1-positive lymphocytes in the tumor microenvironment are an independent prognostic factor in follicular lymphoma. Hum Pathol. 2011;42:552-7.
22. Carreras J, Lopez-Guillermo A, Roncador G, et al. High numbers of tumor-infiltrating programmed cell death 1-positive regulatory lymphocytes are associated with improved overall survival in follicular lymphoma. J Clin Oncol. 2009;27:1470-6.
23. Green MR, Monti S, Rodig SJ, et al. Integrative analysis reveals selective 9p24.1 amplification, increased PD-1 ligand expression, and further induction via JAK2 in nodular sclerosing Hodgkin lymphoma and primary mediastinal large B-cell lymphoma. Blood. 2010;116:3268-77.
24. Dong H, Zhu G, Tamada K, Chen L. B7-H1, a third member of the B7 family, co-stimulates T-cell proliferation and interleukin-10 secretion. Nat Med. 1999;5:1365-9.
25. Shinohara T, Taniwaki M, Ishida Y, et al. Structure and chromosomal localization of the human PD-1 gene (PDCD1). Genomics. 1994;23:704-6.
26. Hardy B, Yampolski I, Kovjazin R, et al. A monoclonal antibody against a human B lymphoblastoid cell line induces tumor regression in mice. Cancer Res. 1994;54:5793-6.
27. Berger R, Rotem-Yehudar R, Slama G, et al. Phase I safety and pharmacokinetic study of CT-011, a humanized antibody interacting with PD-1, in patients with advanced hematologic malignancies. Clin Cancer Res. 2008;14:3044-51.
28. Armand P, Nagler A, Weller EA, et al. Disabling immune tolerance by programmed death-1 blockade with pidilizumab after autologous hematopoietic stem-cell transplantation for diffuse large B-cell lymphoma: results of an international phase II trial. J Clin Oncol. 2013;31:4199-206.
29. Armand P, Welch S, Kim HT, et al. Prognostic factors for patients with diffuse large B cell lymphoma and transformed indolent lymphoma undergoing autologous stem cell transplantation in the positron emission tomography era. Br J Haematol. 2013;160:608-17.
30. Lesokhin A, Ansell S, Armand P, et al. Preliminary results of a phase I study of nivolumab (BMS-936558) in patients with relapsed or refractory lymphoid malignancies. 56th American Society of Hematology (ASH) Annual Meeting; Dec 6–9, 2014; San Francisco, Calif; abstr 291.
31. Westin JR, Chu F, Zhang M, et al. Safety and activity of PD1 blockade by pidilizumab in combination with rituximab in patients with relapsed follicular lymphoma: a single group, open-label, phase 2 trial. Lancet Oncol. 2014;15:69-77.
32. Ansell SM, Lesokhin AM, Borrello I, et al. PD-1 blockade with nivolumab in relapsed or refractory Hodgkin’s lymphoma. N Engl J Med. 2015;372:311-9.
33. Moskowitz A, Ribrag V, Michot J-M, et al. PD-1 blockade with the monoclonal antibody pembrolizumab (MK-3475) in patients with classical Hodgkin lymphoma after brentuximab vedotin failure: preliminary results from a phase Ib study (KEYNOTE-013). 56th ASH Annual Meeting; Dec 6–9, 2014; San Francisco, Calif; abstr 290.
34. Antonia SJ, Brahmer JR, Gettinger SN, et al. Nivolumab (anti-PD-1; BMS-936558, ONO-4538) in combination with platinum-based doublet chemotherapy (PT-DC) in advanced non-small cell lung cancer (NSCLC). American Society of Clinical Oncology (ASCO) 2014 Annual Meeting; May 30–Jun 3, 2014; Chicago, Ill; abstr 8113.
35. John LB, Devaud C, Duong CP, et al. Anti-PD-1 antibody therapy potently enhances the eradication of established tumors by gene-modified T cells. Clin Cancer Res. 2013;19:5636-46.
36. John LB, Kershaw MH, Darcy PK. Blockade of PD-1 immunosuppression boosts CAR T-cell therapy. Oncoimmunology. 2013;2:e26286.
37. Rossille D, Gressier M, Damotte D, et al. High level of soluble programmed cell death ligand 1 in blood impacts overall survival in aggressive diffuse large B-cell lymphoma: results from a French multicenter clinical trial. Leukemia. 2014;28:2367-75.