Renal Cancer Management in a Patient With Chronic Kidney Disease
A 69-year-old man presented in the urology clinic for evaluation of bilateral renal masses, discovered incidentally during routine exams for follow-up of his chronic kidney disease.
Figure: CT scan showing an enhancing solid lesion in the lateral cortex of the right mid kidney (white arrow) and a focal lesion in the lateral cortex of the left mid kidney (yellow arrow).
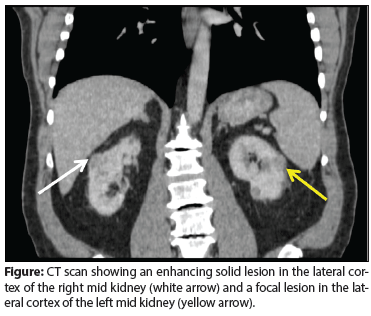
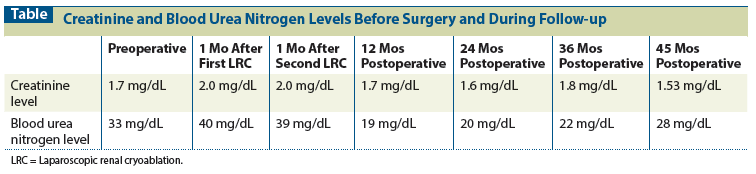
Table: Creatinine and Blood Urea Nitrogen Levels Before Surgery and During Follow-up
The Case: A 69-year-old man presented in the urology clinic for evaluation of bilateral renal masses, discovered incidentally during routine exams for follow-up of his chronic kidney disease. The patient also has a history of type 2 diabetes, hypertension, diabetic neuropathy, and benign prostatic hyperplasia. He has a family history of renal cysts and prostate cancer.
A CT scan revealed bilateral renal masses. On the right side, a mass was discovered in the lateral cortex of the mid kidney that measured approximately 2.7 × 1.8 cm in the cross-section and 3.2 cm in length; it appeared to be solid and heterogeneously enhanced (Figure). On the left side, a focal lesion in the lateral cortex of the mid kidney, measuring approximately 2.2 × 1.5 cm in the maximal cross-section and 2 cm in length, demonstrated enhancement consistent with a solid lesion (see Figure). Six other lesions in the left kidney, 1 cm or smaller in size, were identified; most of them appeared to enhance with intravenous contrast and had soft-tissue attenuation. The differential diagnosis included renal cell carcinoma (RCC) and metastatic disease. The patient’s other organs appeared to be normal.
Treatment options were discussed with the patient, who opted for ablative therapy. Ultimately, bilateral staged renal cryoablation was the treatment option elected by the patient. Laparoscopic renal cryoablation (LRC) was performed on the right side (for the larger tumor) and then on the left side 1 month after initial therapy. The comparison between preoperative and 6 months’ postoperative creatinine and blood urea nitrogen levels is shown in the Table. Laparoendoscopic single-site cryoablation was performed with two cycles of freeze and thaw, and biopsies of the lesions were performed between the first and second freeze cycles, without compromising histologic evaluation, as described in our previous studies.[1] Blood loss during the procedure was minimal (< 50 mL for each procedure) and no complications were reported. The patient had 1 day of hospitalization for each treatment. Pathologic evaluation of the right-side biopsy specimens revealed papillary RCC. On the left side, clear-cell RCC with a Fuhrman nuclear grade of 2 was identified.
Follow-up visits were scheduled every 6 months, and imaging was performed when indicated by clinical evaluation. In a period of 43.5 months of follow-up, there was no suspicion of recurrence and renal function was stable.
Discussion
There are at least two major mechanisms by which cryoablation causes tumor cell death: The first is intracellular ice formation, which leads to mechanical trauma and cellular dehydration with associated osmotic damage[1]; the second is thawing, which results in a decrease in extracellular osmolality as the ice melts, and which can cause an influx of water into cells, leading to cell swelling and bursting. In addition, damage caused to the endothelial blood vessels around the tumor area results in thrombus activation and decreased perfusion in the targeted area, inducing ischemia in remaining/persistent tumor cells.[1]
When working with patients with special conditions, such as chronic renal insufficiency, Von Hippel–Lindau syndrome, bilateral tumors, or large tumor size, treatment decision making must balance oncologic outcomes with the treatment requirements of the patient’s clinical condition.[2-5] Nephron-sparing surgeries and minimally invasive procedures are becoming more popular in the treatment of these patients, since these techniques have fewer perioperative complications but oncologic outcomes comparable to those seen with open nephrectomy.[6-9]
Preservation of renal function without compromising oncologic control is the foundation for nephron-sparing surgery.[10] The current gold standard for small renal masses is partial nephrectomy; however, the technical challenges of the laparoscopic procedure have been associated with high rates of perioperative complications and renal ischemia-reperfusion injury.[11] LRC has demonstrated low intraoperative complication rates without the need for hilar clamping, as well as positive oncologic outcomes.[2,12,13] Oncologic outcomes must be the priority in patients treated for renal cancer.
The recently published American Urological Association (AUA) guidelines for the management of T1 RCC show a recurrence-free survival of 90.6% with cryoablation.[10] Long-term outcomes of LRC were reported by Aron et al, with specific survival rates of 92% at 5 years and 83% at 10 years for patients with renal tumors.[12]
Minimally invasive procedures have been advancing in renal cancer management, with comparable oncologic outcomes; however, whether there is a loss in renal function after the treatment is still debated in the literature. Partial nephrectomy provides equivalent oncologic results, while also preserving renal function and thereby limiting morbidity and cardiovascular mortality related to chronic kidney disease.[14,15] Despite these significant advances in the treatment and understanding of small renal masses, partial nephrectomy is an underused procedure because it is challenging to perform laparoscopically and robotically.
Cryoablation of renal tumors shows similar function and superior perioperative outcomes compared with partial nephrectomy in patients with small renal masses in a solitary kidney.[16] Based on recent data, patients with challenging conditions-such as a solitary kidney, chronic kidney disease, bilateral tumors, or multiple synchronic renal tumors-can benefit from the use of LRC, as demonstrated in our case.
Overall, LRC is an effective nephron-sparing surgical therapy that allows for the preservation of kidney function and has similar rates of surgical complications and better oncologic outcomes compared with other minimally invasive surgical modalities for the treatment of small renal masses. Patients who have a higher probability of tumor recurrence or impairment of renal function should consider LRC, since this technique makes it possible to avoid surgical dissection of renal hilum, thereby facilitating future procedures, should these prove necessary.
Financial Disclosure:The authors have no significant financial interest or other relationship with the manufacturers of any products or providers of any service mentioned in this article.
References:
1. Maccini M, Sehrt D, Pompeo A, et al. Biophysiologic considerations in cryoablation: a practical mechanistic molecular review. Int Braz J Urol. 2011;37:693-6.
2. da Silva RD, Jaworski P, Gustafson D, et al. How I do it: laparoscopic renal cryoablation (LRC). Can J Urol. 2014;21:7574-7.
3. da Silva RD, Molina WR, Gustafson D, et al. Large renal mass: a challenge for the urologist. Oncology (Williston Park). 2014;28:320, 324.
4. Mattei J, da Silva RD, Sehrt D, et al. Targeted therapy in metastatic renal carcinoma. Cancer Lett. 2014;343:156-60.
5. da Silva RD, Gustafson D, Nogueira L, et al. Targeted therapy for metastatic renal carcinoma: an update. J Kidney Cancer VHL. 2014;1:11.
6. Kaouk JH, Autorino R, Kim FJ, et al. Laparoendoscopic single-site surgery in urology: worldwide multi-institutional analysis of 1076 cases. Eur Urol. 2011;60:998-1005.
7. Autorino R, Kaouk JH, Yakoubi R, et al. Urological laparoendoscopic single site surgery: multi-institutional analysis of risk factors for conversion and postoperative complications. J Urol. 2012;187:1989-94.
8. Bove P, Iacovelli V, De Nunzio C, et al. Critical review of laparoendoscopic single-site surgery versus multiport laparoscopy in urology. Arch Esp Urol. 2012;65:348-56.
9. Irwin BH, Cadeddu JA, Tracy CR, et al. Complications and conversions of upper tract urological laparoendoscopic single-site surgery (LESS): multicentre experience: results from the NOTES Working Group. BJU Int. 2011;107:1284-9.
10. Campbell SC, Novick AC, Belldegrun A, et al. Guideline for management of the clinical T1 renal mass. J Urol. 2009;182:1271-9.
11. Fergany AF, Hafez KS, Novick AC. Long-term results of nephron sparing surgery for localized renal cell carcinoma: 10-year followup. J Urol. 2000;163:442-5.
12. Aron M, Kamoi K, Remer E, et al. Laparoscopic renal cryoablation: 8-year, single surgeon outcomes. J Urol. 2010;183:889-95.
13. da Silva RD, Molina WR, Kim FJ. Crioablacao Laparoscopica para tumors renais T1a e T1b: Analise de Resultados. Int Braz J Urol. 2013;39(suppl 1):147.
14. Izzedine H, Méjean A, Escudier B. Kidney function and renal cancer surgery. Bull Cancer. 2014;101:151-66.
15. Russo P. Oncological and renal medical importance of kidney-sparing surgery. Nat Rev Urol. 2013;10:292-9.
16. Panumatrassamee K, Kaouk JH, Autorino R, et al. Cryoablation versus minimally invasive partial nephrectomy for small renal masses in the solitary kidney: impact of approach on functional outcomes. J Urol. 2013;189:818-22.