Retroperitoneal Neuroblastoma Causing Urinary Obstruction in a 5-Month-Old Boy
The patient is a 5-month-old Caucasian boy with no developmental abnormalities who presented Christmas Eve 2004 to his pediatrician with increasing fussiness, emesis, and inability to tolerate oral intake. He had a temperature of 100.2°F but otherwise normal vital signs. Physical exam at that time revealed a distended abdomen. He was sent home with a diagnosis of viral gastroenteritis.
SECOND OPINION
Multidisciplinary Consultations on Challenging Cases
The University of Colorado Health Sciences Center holds weekly second opinion conferences focusing on cancer cases that represent most major cancer sites. Patients seen for second opinions are evaluated by an oncologist. Their history, pathology, and radiographs are reviewed during the multidisciplinary conference, and then specific recommendations are made. These cases are usually challenging, and these conferences provide an outstanding educational opportunity for staff, fellows, and residents in training.
The second opinion conferences include actual cases from genitourinary, lung, melanoma, breast, neurosurgery, and medical oncology. On an occasional basis, ONCOLOGY will publish the more interesting case discussions and the resultant recommendations. We would appreciate your feedback; please contact us at second.opinion@uchsc.edu.
E. David Crawford, MD
Al Barqawi, MD
Guest Editors
University of Colorado Health Sciences Center
Univeristy of Colorado Cancer Center Denver, Colorado
The patient is a 5-month-old Caucasian boy with no developmental abnormalities who presented Christmas Eve 2004 to his pediatrician with increasing fussiness, emesis, and inability to tolerate oral intake. He had a temperature of 100.2°F but otherwise normal vital signs. Physical exam at that time revealed a distended abdomen. He was sent home with a diagnosis of viral gastroenteritis.
Over the next 2 days, he continued to be fussy, developed diarrhea, and was not making wet diapers. He returned to the hospital December 27, 2004, and his exam was now significant for a palpable midline abdominal mass. A catheter was placed and drained 700 mL of urine. After draining the bladder, there was still a palpable abdominal mass slightly off midline to the left. The radiographic findings warranted surgical exploration.
Radiographic Findings
FIGURE 1
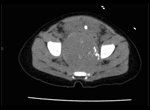
CT Scan of Pelvis
A computed tomography (CT) scan of the abdomen and pelvis was obtained (Figure 1). This revealed a large retroperitoneal pelvic mass left of midline with calcified densities.
Dr. Jesse N. Mills: Dr. Harned, would you please comment on the radiographic findings in this case?
Dr. Roger K. Harned: In the setting of a clinically palpable mass, a CT scan of the pelvis was obtained. This was performed with administered intravenous and oral contrast material with limited non-contrast-enhanced images of the pelvis. The examination showed a 4.8-cm (craniocaudual) X 5.2cm (anteroposterior) X 4.6-cm (transverse) soft-tissue mass filling the lower pelvis, anterior to and inseparable from the sacrum with some protrusion into the left sacrosciatic notch. The bladder, containing a Foley catheter, was displaced anteriorly and superiorly by the mass. There was no bony erosion or expansion of the sacral foramina, and the sacrum was normally formed without cleft or segmentation anomaly.
The mass was of soft-tissue density, contained scattered punctate and linear calcifications, and enhanced homogenously after administration of intravenous contrast material. There were no associated fluid collections, and the mass contained no fat density material. The retroperitoneum was free of mass or adenopathy; adrenal glands were normal; and the liver enhanced homogeneously without evidence of metastatic disease.
FIGURE 2
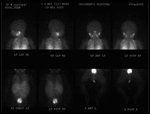
I-123 MIBG Scan
Differential diagnosis based on the CT scan would include sacrococcygeal teratoma, rhabdomyosarcoma, neuroblastoma, and neurofibroma. The absence of associated bony abnormality and presence of scattered calcifications suggest neuroblastoma as the first choice. An I-123 metaiodobenzylguanidine (MIBG) scan was subsequently performed (Figure 2). The tumor demonstrated marked avidity for the radiopharmaceutical with no abnormal activity beyond the primary mass. This abnormal accumulation is specific for a tumor of adrenergic origin. Again, neuroblastoma is the most likely diagnosis, although a pelvic pheochromocytoma could also have this appearance.
Surgical Considerations
Dr. Mills: Dr. Hendrickson, how did you plan your surgical approach and what are the technical considerations of this case?
Dr. Richard J. Hendrickson: My differential diagnosis prior to surgery was a neuroblastoma vs a sacrococcygeal teratoma, although one must favor a neuroblastoma based on the calcifications noted on CT scan. My plan was to perform a diagnostic laparoscopy with biopsy of the tumor. On the CT scan, I noted that the Foley balloon was anterior to this pelvic mass, which I thought would be amenable to a laparoscopic biopsy.
After the induction of anesthesia, a midline palpable mass was noted to extend from the symphysis pubis to the umbilicus. I was able to palpate the Foley balloon within this structure. Therefore, I decided to make a supraumbilical incision to avoid injury to the bladder. Also, on rectal exam, the mass was palpable. After placing my supraumbilical port (5 mm), two additional 5-mm ports were placed in the left and right abdominal wall. We noted a large cystic structure extending from the umbilicus down into the pelvis, presumably the bladder, although it looked abnormal and I thought possibly had calcifications in the wall.
During diagnostic laparoscopy, the pelvic tumor could not be safely identified. Dissection was performed down to the bifurcation of the internal and external iliac arteries. We clearly saw these vessels, as well as the ureters. Next, we instilled sterile water into the Foley and observed the midline cystic structure expand, confirming that this was indeed the bladder. It seemed odd to me that on CT scan the Foley balloon was juxtaposed to the pelvic tumor, and I could see the Foley balloon yet I could not identify the tumor. Again, the wall of the bladder appeared to have calcifications, and I wondered whether this was a bladder tumor rather than a pelvic/sacral tumor.
Therefore, I consulted Dr. Mingin for an intraoperative cystoscopy. Based on his findings, I aborted the laparoscopic approach, closed, and after further discussion with Oncology, Radiology, and Urology, decided to approach the tumor via a posterior sagittal approach with a coccygectomy the following day.
Dr. Mills: Dr. Mingin, you were consulted intraoperatively based on the fact that Dr. Hendrickson did not visualize the tumor laparoscopically. Please comment on the cystoscopic evaluation.
Dr. Gerald C. Mingin: First of all, this was a difficult cystoscopy secondary to the anterior displacement of the prostatic urethra and bladder neck by the tumor. Once I entered the bladder, I saw a pedunculated vascularized lesion emanating from the left lateral wall and dome of the bladder. I was able to use a resectoscope to take a deep biopsy of this lesion.
Pathology
Dr. Mills: Dr. Lovell, please review the pathology for us.
FIGURE 3
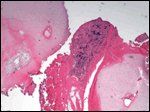
Tumor Infiltrating Soft Tissue
Dr. Mark A. Lovell: The resectoscope biopsy contained urothelium and subjacent stroma but no recognizable tumor. A bone marrow aspirate was also negative for tumor. However, diagnostic tissue was obtained from the coccygectomy specimen. Figure 3 shows a small blue cell tumor infiltrating the soft tissue between the cartilaginous vertebrae without an appreciable Schwannian stromal component (Schwannian stroma-poor). At higher power (Figure 4), the neuropil background, in which scattered pseudorosettes are evident, confirms the diagnosis of neuroblastoma. Of note, no calcifications are seen in the sections examined. Electron microscopy performed as a subsequent quality assurance measure confirmed the presence of neurosecretory granules.
The International Neuroblastoma Pathology Classification[1,2] provides reproducible and clinically significant criteria to categorize neuroblastic tumors as having favorable or unfavorable histology. The first step is to determine if Schwannian stroma (the supporting component of nerves) is present in an appreciable amount. A tumor that is Schwannian stroma-poor (as in this case) is a neuroblastoma, as opposed to a ganglioneuroblastoma, which is stroma-rich.
FIGURE 4
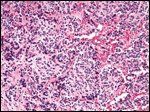
Tumor With Pseudorosettes
Next, the tumor's degree of differentiation is assessed. This ranges from undifferentiated (meaning that the small blue cell tumor's neuroblastic character is only evident with immunohistochemical, ultrastructural, or cytogenetic studies) to poorly differentiated (the most common classification, as seen in this case), in which neuropil is evidence of neuroblastic differentiation, to differentiating tumors with at least 5% ganglion cells.
The next step is to assess the number of mitotic and karyorrhectic (apoptotic) cells to determine the mitotic-karyorrhectic index (MKI). The MKI may be low (< 100), medium (100-200) or high (> 200) relative to 5,000 cells, with high MKI always being unfavorable. In this case the MKI was low. Finally, the patient's age is factored in (generally younger or older than 18 months, but in the case of differentiating tumors with low MKI, the breakpoint is 5 years), with older age being unfavorable. Combining these four factors in this case categorizes the tumor as having a favorable histology.
Staging
Dr. Mills: Dr. Albano, how is neuroblastoma staged and what anatomic, radiographic, or serologic data do you need to stage?
Dr. Edythe A. Albano: At presentation, we primarily considered neuroblastoma and a sacrococcygeal germ cell tumor in our differential because of the pelvic location, intimate association with the sacrum, and calcifications within the tumor. Although rhabdomyosarcomas arising in the bladder and prostate can cause urinary tract obstruction, calcifications are not usually seen within the tumor. Additionally, their location is more anterior in the pelvis. Germ cell malignancies in young children are usually endodermal sinus tumors (yolk sac tumors) and are associated with elevated alpha-fetoprotein (AFP). When this patient's AFP returned within the normal range for his age, we moved neuroblastoma to the top of our differential diagnosis list.
Ultimately, the urinary catecholamines came back elevated, consistent with the diagnosis of neuroblastoma. However, this is a study sent to a reference laboratory and the results are generally not available for a week or more. Additionally, a finding of elevated urinary catecholamines is not adequate for making a diagnosis of neuroblastoma and gives no information as to the likely biologic behavior of the tumor-information essential in treatment planning. Lastly, urinary catecholamines are not always positive in patients with neuroblastoma. A tissue diagnosis is essential for the diagnosis of neuroblastoma. Biopsy of the tumor mass ultimately provided us with the pathologic diagnosis and material to evaluate biologic variables. Staging of neuroblastoma is based on evaluation of the size of the primary tumor and presence or absence of metastatic disease. The International Neuroblastoma Staging System (INSS) is shown in Table 1.[3]
Given the infiltration of the tumor across the midline, this patient had at least stage 3 disease. Subsequent CT imaging of the neck, chest, and abdomen, bilateral bone marrow aspirates and biopsies, as well as bone and MIBG scans ruled out metastatic disease and confirmed the stage as INSS 3.
Goals of Surgery
Dr. Mills: Dr. Petty, what are the goals of surgical therapy in this disease and how often is primary surgical cure possible?
Dr. John K. Petty: Complete surgical resection should always be the goal for neuroblastoma. However, due to the neural crest origin of these tumors, they are often intimately involved with the great vessels of the mediastinum, retroperitoneum, or pelvis. Thus, the goal in the operating room is excision of gross tumor with complete vascular dissection and resection of regional lymph nodes. According to the INSS, neuroblastoma is staged in part based on the completeness of surgical resection. Localized tumors should be resected primarily. Stage 1 and stage 2 patients with favorable biologic characteristics may be treated with primary resection alone. These patients enjoy > 85% survival.
Unfortunately, 80% to 90% of neuroblastoma patients present with advanced disease (stage 3 and 4). In these patients, primary resection is typically postponed until neoadjuvant chemotherapy is completed. This treatment strategy for advanced disease increases resectability by nearly twofold.[4] The current patient has a very large tumor in proportion to the size of his true pelvis, precluding primary surgical resection. It will be interesting to see how his tumor decreases in size in response to chemotherapy. His tumor response will dictate whether his primary resection is performed through an abdominal, posterior sagittal, or combined approach.
Dr. Albano: Neuroblastoma exhibits a wide range of biologic behaviors, from spontaneous regression to rapid progression and death despite aggressive therapy. Patient age at diagnosis, stage of disease, and biologic variables within the tumor enable us to categorize patients into low-, intermediate-, and high-risk categories and tailor their therapy accordingly. Currently, histology (favorable vs unfavorable), MYCN status (myc myelocytomatosis viral-related oncogene, neuroblastoma derived-amplified vs unamplified) and DNA index (less than or greater than 1) are the biologic parameters that best predict behavior of the tumor as well as response to therapy and eventual disease-free survival. Early stage, age less than 1 year, unamplified MYCN, hyperdiploidy, and favorable histology are generally associated with more favorable outcomes and the need for less intensive therapy.
This patient who had favorable biologic characteristics, age less than 1 year, and stage 3 disease was characterized as intermediate-risk. He was enrolled in the Children's Cancer Group (CCG) cooperative trial for the treatment of intermediate-risk neuroblastoma and will receive four cycles of moderate-dose chemotherapy that include the active agents carboplatin, etoposide, doxorubicin, and cyclophosphamide. He will then undergo evaluation for tumor response. Surgical resection will take place at that time, if feasible. Radical surgery is not recommended. If surgery successfully removes all gross disease, no further therapy will be administered. If surgery is not feasible, four more cycles of therapy will be administered, and surgery will then be undertaken. Radiation is generally reserved for patients with unfavorable biology.
Differential Diagnosis
Dr. Mills: Dr. Furness, what is your differential diagnosis of a pelvic tumor in an infant?
Dr. Peter D. Furness: There are a few entities one must consider. Sacralcoccygeal teratoma is one possibility. Usually these tumors present with a visible bulge in the sacrum. Teratomas entirely within the pelvis can be palpated as a solid posteriorly placed mass on rectal exam. Myelomeningocele must also be considered in the differential diagnosis. Often, there is a cutaneuos finding of sacral dimpling or hair tuft. Further, there is a serous membrane covering myelomeningocele. A retrorectal mass that is cystic to palpation differentiates a myelomeningocele from a sacralcoccygeal teratoma. Rhabdomyosarcoma is another possibility. The genitourinary tract represents 21% of all rhabdosarcomas but is still not as common as neuroblastoma. Neuroblastoma is certainly a major consideration for any differential diagnosis of solid tumors in an infant. The finding of calcifications in the tumor is helpful in making this diagnosis.
Epidemiology
Dr. Mills: How often is neuroblastoma seen in the pelvis?
Dr. Mingin: The pelvis is certainly not the most common site. One recent series from France looked at 52 cases over a 10-year period and found 40 of the cases to be abdominal, 8 to be thoracic, 1 cervical, and 3 in the pelvis.[5] There are 6 reported cases in the literature of neuroblastoma involving the bladder.[6]
Dr. Mills: Dr. Koyle, how often have you seen a pelvic tumor cause urinary obstruction?
Dr. Martin A. Koyle: There have been two other case reports in the literature describing pelvic tumors in the neonate causing bladder outlet obstruction.[7] In the adult, with cervical and prostate cancer, it is far more common. In my practice, I have never seen bladder outlet obstruction from pelvic tumors originating outside of the genitourinary tract. Intrinsic genitourinary tract tumors such as rhabdomyosarcoma of the prostate can certainly cause bladder outlet obstruction.
Prognosis
Dr. Mills: Dr. Albano, based on the information so far, what prognosis do you give this boy?
Dr. Albano: The current treatment approach is based on previous CCG studies. Matthay and colleagues have reported the CCG experience in 143 infants with intermediate-risk disease treated with moderate-dose chemotherapy and radiation therapy for gross residual disease following delayed surgery. The 4-year event-free survival for patients with Evans stage III disease, normal MYCN, favorable Shimada, and normal ferritin was 100%. Infants with Evans stage III and at least one favorable biologic feature had an event-free survival of 90% and overall survival of 93% with this treatment approach.
Recommendations
Dr. Mills: This boy is now 7 months old and has been diagnosed with stage III neuroblastoma based on an excisional biopsy. He has undergone surgical biopsy via a posterior sagittal approach to confirm the diagnosis. He is currently undergoing chemotherapy and will return to the operating room at the end of his fourth cycle of chemotherapy. Neuroblastoma involving the bladder and causing urinary obstruction has only been reported a few times. This boy's overall prognosis remains favorable. A multidisciplinary approach involving pediatric surgery, oncology, and urology, with diagnostic and prognostic information from radiology and pathology is essential in providing the child with the best possible outcome.
Financial Disclosure:The authors and participants in this conference have no significant financial interest or other relationship with the manufacturers of any products or providers of any service mentioned in this article.
References:
1. Shimada H, Ambros IM, Dehner LP, et al: Terminology and morphologic criteria of neuroblastic tumors: Recommendations by the International Neuroblastoma Pathology Committee. Cancer 86:349-363, 1999.
2. Shimada H, Ambros IM, Dehner LP, et al: The International Neuroblastoma Pathology Classification (the Shimada system). Cancer 86:364-372, 1999.
3. Brodeur GM, Seeger RC, Barrett A, et al: International criteria for diagnosis, staging, and response to treatment in patients with neuroblastoma. J Clin Oncol 6:1874-1881, 1988.
4. Adkins ES, Sawin R, Gerbing RB, et al: Efficacy of complete resection for high-risk neuroblastoma: A Children's Cancer Group study. J Pediatr Surg 39:931-936, 2004.
5. Michalowski MB, Rubie H, Michon J, et al: [Neonatal localized neuroblastoma: 52 cases treated from 1990 to 1999]. Arch Pediatr 11:782-788, 2004.
6. Entz-Werle N, Marcellin L, Becmeur F, et al: The urinary bladder: An extremely rare location of pediatric neuroblastoma. J Pediatr Surg 38:E10-12, 2003.
7. Pinter A: Bladder outlet obstruction in the neonate. Acta Paediatr Hung 25:355-362, 1984.
Late Hepatic Recurrence From Granulosa Cell Tumor: A Case Report
Granulosa cell tumors exhibit late recurrence and rare hepatic metastasis, emphasizing the need for lifelong surveillance in affected patients.